For decades, water consumption has been increasing worldwide and water shortages are occurring in more and more regions. We, too, need this resource at our production plants and in our healthcare facilities and want to handle it responsibly. We work with management systems and control systems globally to ensure that water quality meets internal and external regulatory requirements.
Our goals and ambitions
It is our ambition that water in any area of our business can be used safely and not harm the health of our patients and employees, and that it is sufficiently available at all times. We further aim to avoid unnecessarily polluting the sources from which we obtain water or into which we discharge our wastewater.
Our approach
We use local management systems, process owners, and operating procedures to ensure that the respective local guidelines on water and wastewater are strictly adhered to. Water management measures consider a reduction in water and wastewater volumes, and monitor the quality and authorized withdrawal of water and discharge of wastewater.
Fresenius continuously reviews national and international regulations on water management. The internal principles, guidelines, and standard operating procedures – which contain instructions for the responsible handling of water, including the control of wastewater – are adapted to the applicable regulatory requirements. Our water management is closely linked to our hygiene management. Depending on the business unit, either environmental or hygiene experts ensure that internal guidelines and external regulations are adhered to.
Water usage and withdrawal
In production, water is used for sterilization and cooling processes, as a component in the production of medical products, and for hygiene procedures. The water used for our products, e. g., for infusion solutions such as sodium chloride, must meet stringent quality requirements to ensure product quality and patient safety.
For our healthcare facilities, a sufficient supply of fresh water is central to patient well-being and hygiene. Most of the water withdrawal is from municipal water supplies. The largest freshwater users are rehabilitation clinics with therapy pools, e. g., in the orthopedics department, and facilities that sterilize used medical instruments.
In 2023, Fresenius withdrew a total of 15.1 million m3 of water (2022: 15.6 million m3). Over the last years, a relative reduction in water withdrawal was achieved, both in relation to sales and to full-time equivalents (FTE). In our healthcare facilities, water withdrawal depends on the number of patients treated in hospitals. The previous years were further impacted by an increased demand for sterilization and hygiene. Reduction in water withdrawal compared to last year is based on water saving measures.
Absolute water withdrawal
Download(XLS, 35 KB)| m3 in millions | 2023 | 2022 | 2021 |
|---|---|---|---|
| Healthcare products market segment | 9.9 | 10.4 | 10.1 |
| Healthcare services market segment | 5.2 | 5.2 | 4.9 |
| Total | 15.1 | 15.6 | 15.0 |
Relative water withdrawal
Download(XLS, 35 KB)| in m3 | 2023 | 2022 | 2021 |
|---|---|---|---|
| Water withdrawal / €1 million sales | 671 | 718 | 797 |
| Water withdrawal / FTE | 92.0 | 98.1 | 97.0 |
Water withdrawal by source
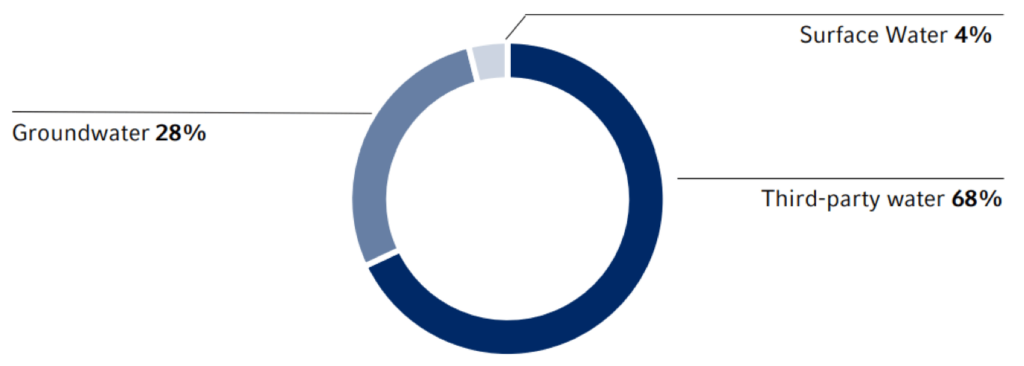
Measures to reduce water consumption
Some manufacturing sites are reusing water, e. g., by using condensate water from installed air handling units or in steam condensate recovery systems. In the reporting year, implementation of several projects to save water was started at the production sites. Wastewater treatment systems and recycling programs, for example, aim to minimize wastewater and use resources more sustainably. We have also optimized cleaning and sterilization processes at several locations. Positive effects on water consumption are expected in 2024.
Due to the material significance of fresh water use for compliance with hygiene measures and thus patient safety in our healthcare facilities, no significant reductions in water withdrawal are made. Due to internal requirements regarding drinking water quality, we do not reuse water or use gray water – i. e., treated water from showers or washbasins.
Water quality
We have implemented applicable risk management procedures in all facilities that come into action if impurities are detected or if the quality of water is not compliant with standards set – and established dedicated reporting lines. The local government is informed of any detected critical deviations from local drinking water provisions. In Germany, some of our clinics are designated as testing centers for local drinking water quality. In this way, we support not only the safety of our patients, but also that of the surrounding population and the municipalities that supply us with drinking water.
In the case of contaminated fresh water from the public network, our clinics have the option of connecting additional water treatment modules upstream of the hospital’s own network in addition to its own treatment facilities. All hospitals have contingency plans in place in the event of supply bottlenecks to ensure healthcare for patients.
Water discharge
Water discharges are locally managed at the sites in accordance with applicable local regulations. In production, water discharge by quantity is regularly reported to global EHS (Environmental, Health, and Safety) in accordance with internal standards and guidelines. In addition, Fresenius Kabi has been a member of the Antimicrobial Resistance (AMR) Industry Alliance (AMRIA) since 2020 and has been actively involved in the association’s governing bodies since 2021. The business segment is working on the introduction of the AMR Industry Alliance’s Common Antibiotic Manufacturing Framework (CAMF). For further information, please refer to the Group Non-financial Report 2022 on page 208.
In 2022, AMRIA and BSI Standards Limited released the Antibiotic Manufacturing Standard, providing guidance to manufacturers on responsible antibiotic production. The goal of this standard is to minimize the risk of developing antibiotic resistance and reduce aquatic ecotoxicity in the environment resulting from the manufacturing of human antibiotics. The standard complements the already high production quality and safety management at our production sites. A pivotal component of the approach involves the use of a risk-based methodology to evaluate and control the waste streams generated during antibiotic manufacturing.
The implementation, which began in 2022, involved the introduction of a comprehensive quantification mass balance template by Fresenius Kabi. The template’s function is to assist antibiotic manufacturing sites in determining antibiotic concentrations in manufacturing wastewater discharge and conducting gap analyses, with the overarching goal of aligning with the Predicted No-Effect Concentrations (PNEC) set forth by the AMRIA. PNEC represents the concentration level of a substance in the environment below which no adverse effects are expected.
Furthermore, a dedicated communication channel has been established to connect local sites with the global EHS team. This initiative fosters continuous alignment with the Antibiotic Manufacturing Standard, ensuring ongoing adherence and improvement in the future.
Identification and management of water risks
We analyze water availability using the World Resources Institute’s Aqueduct Water Risk Atlas, which contains information on current and future water risks at specific locations. We have identified manufacturing sites that are in areas with extremely high or high risk of water scarcity. At these sites, efficient water management is especially important to ensure water availability for production and to prevent negative impact on the local water situation as far as possible.
Manufacturing plants are requested to conduct a climate risk assessment including water risks such as floods, droughts, or heavy rain and set up measures in case a risk is identified.
In the hospital sector, evaluation of water risks is carried out as part of Group risk management.
Contact
Fresenius SE & Co. KGaA
Group ESG
sustainability@fresenius.com

