For decades, water consumption has been increasing worldwide and water shortages are occurring in more and more regions. We too need water, both at our production plants and in our healthcare facilities. We therefore handle this scarce and vital resource responsibly. We work with management systems and control systems globally to ensure that water quality meets internal and external regulatory requirements so it can be used safely during production, in processes, and in our healthcare facilities. The health of our patients and employees must be protected. The aim of our water management is therefore not only to ensure the highest quality and sufficient availability of freshwater but also to avoid unnecessary polluting the sources from which we obtain water or into which we discharge our wastewater. Water withdrawal for the Fresenius Group has been surveyed annually since 2016 as a part of non-financial reporting.
Our approach
Fresenius continuously reviews national and international regulations on water management. This ensures that internal principles, guidelines, and standard operating procedures are always up to date or often go beyond regulatory requirements, e. g., within the framework of global management handbooks. Depending on the operating activity, either environmental or hygiene experts ensure that internal guidelines and external regulations are adhered to.
At our clinics and hospitals, most of the water withdrawal is from municipal water supplies. We have implemented applicable risk management procedures that come into action if impurities are detected or if the quality of water is not compliant with standards set. Further, dedicated reporting lines provide transparency within the business segments. The local government is informed of any detected critical deviations from local drinking water provisions.
In Germany, some of our clinics are designated as testing centers for local drinking water quality. In this way, we support not only the safety of our patients, but also that of the surrounding population and the municipalities that supply us with drinking water.
In production, water is needed for sterilization and cooling processes, as a component in the production of medical products, for hygiene procedures, and as a vital element of our dialysisDialysisForm of renal replacement therapy where a semipermeable membrane – in peritoneal dialysis the peritoneum of the patient, in hemo dialysis the membrane of the dialyzer – is used to clean a patient’s blood. solutions. 69% of water withdrawal in production is sourced from the municipal water supply, 27% through groundwater sources, and 4% from surface water. Fresenius operates in highly regulated markets with regard to hygiene, sterility, and product quality. This ranges from standard operating procedures for risk prevention and maintenance to monitoring strategies, as well as emergency and response management systems with regard to environmental incidents.
Fresenius Medical Care
Large volumes of water are required both in production sites and in dialysis clinics as the dialysis process requires a significant quantity to provide life sustaining care for dialysis patients. It is critical that the water Fresenius Medical Care uses for dialysis is of high quality, which is why the company generally uses municipal water that is treated further in its dialysis clinics.
Progress and measures in 2022
In 2022, Fresenius Medical Care continued to build on the water stress-related assessments that the company has been performing since 2020 with the support of the World Resource Institute’s Aqueduct tool. The most recent water stress analysis in 2021 confirmed that 12% of its dialysis clinics and 7% of the production sites are situated in locations identified by the tool as having an extremely high risk of water stress. The assessment covered 77% of the dialysis clinics and all its production sites. By 2023, Fresenius Medical Care aims to expand the coverage of this analysis to include additional dialysis clinics.
In the reporting year, the business segment focused on further developing the water stress scenario analysis, which it initiated in 2021. The aim of this analysis is to identify areas around the world where water stress levels will increase most by 2030 and 2040. Fresenius Medical Care determined that a considerable number of the existing sites are in locations that are expected to have high or extreme water stress levels by these dates. Most of them are situated in North America which accounts for the largest share of its business. Sites in Europe, Middle East and Africa, Latin America, and Asia-Pacific are also likely to be affected by increasing water stress. Fresenius Medical Care is actively incorporating insights from this analysis into the Group-wide risk management systems to identify, monitor, and mitigate possible risks as early as possible.
Fresenius Kabi
Water is primarily used in production at Fresenius Kabi, e. g., for cooling or in sanitary facilities, and is discharged as wastewater. Some manufacturing sites are reusing water, e. g., by using condensate water from installed air handling units or in steam condensate recovery systems. The business segment also uses water for its products, e. g., for infusion solutions such as sodium chloride. The water used for this purpose must meet stringent quality requirements to ensure product quality and patient safety. Fresenius Kabi’s global environmental standard operating procedures and working instructions include instructions for the responsible handling of water, including the control of wastewater. Each of Fresenius Kabi’s manufacturing sites is required to evaluate its environmental impact, e. g., from water usage and wastewater. Water management measures consider a reduction in water and wastewater volumes, and monitor the quality and authorized withdrawal of water and discharge of wastewater.
Water discharges are locally managed at the sites in accordance with applicable local regulations. Water discharge by quantity is regularly reported to global EHS in accordance with internal standards and guidelines. In addition, Fresenius Kabi has been member of the AMR Industry Alliance since 2020 and has been actively involved in the association’s governing bodies since 2021. The business segment is in the process of implementing the Common Antibiotic Manufacturing Framework (CAMF) of the AMR Industry Alliance. According to the CAMF requirements, wastewater contaminated with antibiotic residues should not be discharged untreated. In 2021, the business segment started to establish corresponding processes and measures at the sites that produce antibiotics. These processes and measures complement the existing internal standards and procedures. For example, systems to control Predicted No-Effect Concentrations (PNEC) were introduced. PNEC values can be used to determine discharge concentration targets for antibiotics that are not expected to cause environmental effects.
Water availability at Fresenius Kabi’s production sites is important to ensure business continuity. The business segment analyzes the water situation using the World Resources Institute’s Aqueduct Water Risk Atlas, which contains information on current and future water risks at specific locations. Fresenius Kabi has identified manufacturing sites that are in areas with extremely high or high risk of water scarcity. At these sites, efficient water management is especially important to ensure water availability for production and to prevent negative impact on the local water situation as far as possible.
Environmental protection, including protection of marine resources, is an important aspect of product development at Fresenius Kabi. Therefore, the business segment selects fish oil suppliers for SMOFlipid® and Omegaven® lipid emulsions for infusion certified by the label Friends of the Sea.
Manufacturing plants are requested to include water stress and other applicable types of water risks such as floods, droughts or heavy rain into their risk assessments and set up measures in case a risk is identified. Manufacturing plants certified according to ISO 14001 are requested to improve their environmental performance, including water, on an annual basis. In addition, national requirements on the handling and use of water are to be followed on a local level. Fresenius Kabi’s Global Competence Cluster (GCC) Energy and Water Management supports the business segment’s manufacturing plants in managing water as a scarce resource. Management practices are being shared among sites and water saving projects are continuously being fostered. Thereby, Fresenius Kabi aims to increase efficiency of water usage.
Progress and measures in 2022
In 2022, the management approach and the governance structure of Fresenius Kabi remained as reported in 2021. Progress focused on conducting water risk assessments which were requested from all manufacturing plants. Sites with high water risks have been asked to develop actions plans to mitigate them. Action plans will be reviewed during global internal EHS audits by Fresenius Kabi’s global EHS function. In addition, the progress of implementation of the CAMF continued with a focus on methods for treating wastewater as well as measuring or calculating concentrations of antibiotics in wastewater.
For example, as part of the GCC’s 2022 campaign to raise awareness of environmental management and generate approaches to improve water and energy management at its sites, the following ideas were submitted for water: The manufacturing plant in Kalyani, India, developed an approach to reduce water consumption by using treated wastewater as water for its cooling towers. If implemented, this can reduce municipal- and groundwater consumption by 32.000 m³ per year. In addition, the manufacturing plant in Uppsala, Sweden, saw potential in the reuse of condensate saving approximately 2.100 m³ water per year. By modernizing water ring vacuum pumps at the manufacturing plant in Kutno, Poland, potentially about 12.000 m³ of water could be saved per year. Fresenius Kabi identified those ideas with the greatest potential for improvement and is working on their implementation at the respective sites.
Fresenius Helios
The focus of water management at the Helios clinics lies on ensuring an uninterrupted supply of water of consistently high quality and on preventing microbiological contamination. The use of water as a resource in healthcare facilities is subject to strict legal requirements both in Germany and in Spain. Rainwater, for instance, can only be used in areas that are not critical for patient safety. Compliance with the respective applicable regulatory requirements, e. g., the Drinking Water Ordinance in Germany, has top priority. In order not to endanger patients, employees, or other people at any time, water management is closely linked to hygiene management.
In the case of contaminated fresh water from the public network, Helios Germany has the option of connecting additional water treatment modules upstream of the hospital’s own network in addition to its own treatment facilities. All Helios Germany and Helios Spain hospitals have contingency plans in place in the event of supply bottlenecks to ensure healthcare for patients.
The company’s own guidelines and specifications determine the hospital-specific procedures. Further internal requirements regarding drinking water quality apply. These must be implemented in all German and Spanish facilities. For these reasons, Fresenius Helios does not reuse water or use gray water – i. e., treated water from showers or washbasins. Helios Spain supports the careful use of water, as there is a water shortage in certain Spanish regions that exposes the country to the risk of increasing desertification.
For the discharge of wastewater, Fresenius Helios in Spain and Germany must comply with strict regional and local legal requirements, which are monitored within the respective wastewater treatment plants. Deviations are reported directly to the hospital concerned and forwarded to all responsible departments through established reporting chains. After evaluating an incident, Fresenius Helios aims to ensure that the requirements are met in future. This is enabled through measures like technical improvements or changes to processes and additional training.
Progress and measures in 2022
In 2022, the management approach and governance structure in the water management area of Fresenius Helios remained as reported in the previous year.
Fresenius Vamed
For Fresenius Vamed, a sufficient supply of fresh water for patient well-being and hygiene is a key element in the planning, construction, and operation of healthcare facilities. The healthcare facilities built by the business segment use construction and sanitation technology that enables optimal water management – adapted to local regulations. At the same time, intelligent water management must under no circumstances undermine hygiene measures or jeopardize the well-being of patients. The largest freshwater users at Fresenius Vamed are rehabilitation clinics with therapy pools, e. g., in the orthopedics department, and facilities that sterilize used medical instruments.
Fresenius Vamed uses local management systems, process owners, and operating procedures to ensure that the respective local guidelines on water and wastewater are strictly adhered to. The internal principles, guidelines, and standard operating procedures are adapted to the applicable regulatory requirements.
Due to the material significance of fresh water use for compliance with hygiene measures and thus patient safety, no significant reductions in water withdrawal are made. A secondary use of water is refrained from due to the hygiene issues that need to be taken into account. In the long term, the business segment aims to achieve constant water withdrawal.
In contrast to industrialized countries with good infrastructure and strict regulation, adequate water supply in developing markets is a major challenge. In the non-European countries where Fresenius Vamed is active in project business, legal requirements for water quality are often not comparable with the high standards required for the operation of a healthcare facility in Europe, for instance. The same applies to the treatment of wastewater. Many projects require the use of freshwater and wastewater treatment plants. The non-European projects are implemented according to the guidelines for water quality of the World Health Organization (WHO). Fresenius Vamed aligns the high-quality standards for the plants to be built with the requirements set by international project financers and implements them during the planning phase.
In project business, Fresenius Vamed ensures that the latest generation of water-saving technologies is used. Future operators and employees receive comprehensive training. In the non-European markets, the business segment also uses fully biological sewage treatment plants in its project business to treat wastewater.
Progress and measures in 2022
In 2022, the management approach and governance structure in the water management area of Fresenius Vamed remained as reported in the previous year. At the healthcare facilities in Austria, work began in the reporting year to examine the installation of water-saving systems. In the first step, a hygienic expert opinion confirmed their suitability. Subsequently, the reduction in consumption resulting from the installation will be calculated in order to be able to evaluate the effectiveness of the measure. The result of the test is expected at the beginning of 2023.
Evaluation1
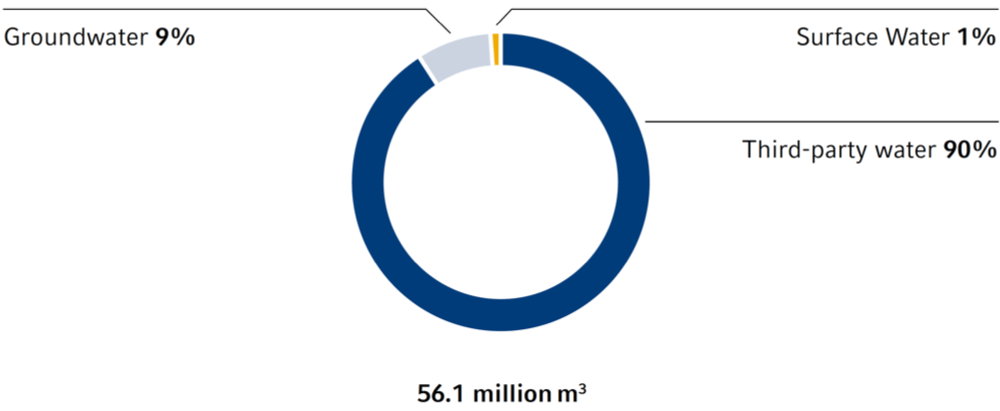
In 2022, Fresenius withdrew a total of 56.1 million m3 of water (2021: 56.4 million m3). Over the last three years, a relative reduction in water withdrawal was achieved, both in relation to sales and to full-time equivalents. Around 90% came from the municipal water supply, while about 9% was sourced from groundwater and 1% from surface water. In the hospital and rehabilitation sector in particular, water is sourced from the municipal water supply. This is due to the strict hygiene regulations and high demands on water quality in healthcare facilities. Furthermore, no incidents were recorded in which the respective environment or the general public were directly harmed.
Water withdrawal Fresenius Group1
Download(XLS, 35 KB)| m3 in millions | 2022 | 2021 | 2020 | 2019 | 2018 |
|---|---|---|---|---|---|
| Fresenius Medical Care2 | 40,5 | 41.4 | 41.7 | 43.2 | 42.1 |
| Fresenius Kabi | 10.4 | 10.1 | 9.7 | 9.5 | 9.7 |
| Fresenius Helios3 | 4.2 | 4.0 | 4.1 | 3.8 | 3.7 |
| Fresenius Vamed | 1,0 | 0.9 | 0.8 | 0.7 | 0.7 |
| Total | 56.1 | 56.4 | 56.2 | 57.2 | 56.2 |
Fresenius Group1 relative water withdrawal
Download(XLS, 35 KB)| in m3 | 2022 | 2021 | 2020 | 2019 | 2018 |
|---|---|---|---|---|---|
| Water withdrawal / € 1 million sales | 1,373 | 1,503 | 1,550 | 1,609 | 1,676 |
| Water withdrawal / FTE | 199.8 | 201.9 | 203.3 | 217.5 | 228.2 |
Water withdrawal by source
In 2022, Fresenius Medical Care’s reported water withdrawal decreased by 2% compared with 2021. This was mainly due to a decrease in the number of treatments the business segment provided.
In addition to the water stress analyses, in 2022, Fresenius Medical Care defined global water-related objectives to supplement those already at a regional level. For example, the company has set itself the global target of developing and implementing sustainable water plans for production sites and dialysis clinics in extremely high water stress areas by 2026. These plans are intended to lay out optimization and improvement measures for the sites in question.
In 2022, Fresenius Kabi asked selected antibiotic-producing sites to prepare a mass balance on antibiotic residues in wastewater. This, together with the measurements carried out as part of a pilot project, leads to enhanced transparency about possible antibiotic residues in wastewater and contributes to the continuous improvement of water management. Water withdrawal at the business segment was 10.4 million m3 in 2022 (2021: 10.1 million m3) which amongst other things increased due to higher production at some sites compared to the previous year. In 2022, several projects to reduce water withdrawal were implemented at manufacturing plants of Fresenius Kabi. Water-saving projects in 2022 included, e. g., installation of ultrafiltration and reverse osmosis system in wastewater treatment plant to reduce wastewater generation and water consumption saving about 24.000 m3 water annually, another site has implemented a system to reduce the discharge of water for injection (WFI) resulting in a saving of about 5.000 m3.
Water withdrawal at Fresenius Helios was 4.2 million m3 in 2022 (2021: 4.0 million m3). Water withdrawal depends on the number of patients treated in hospitals. The previous years were further impacted by an increased demand for sterilization and hygiene.
Water withdrawal per m2 of total operating space
Download(XLS, 35 KB)| 2022 | |
|---|---|
| Helios Germany | 0.73 m3 |
| Helios Spain | 1.0 m3 |
In the business segment Fresenius Vamed, water withdrawal increased compared to the previous year. Total water withdrawal was 1.0 million m³ in 2022 (2021: 0.9 million m³). The increase in consumption was due to a less severe pandemic situation in the reporting year. Specifically, more technical services were again performed in the area of sterile supply, and there was also greater occupancy of healthcare facilities.
1 Newly acquired companies are included in the second year of consolidation, at the latest. If data of the business segments is not available in time, it is extrapolated on the basis of existing data. An adjustment will be made in the next report. Prior-year information was adjusted to conform to the current year’s presentation. Due to rounding, individual numbers and percentages presented in this report may not precisely reflect the absolute figures.
2 Fresenius Medical Care water figures include the water withdrawal of its production sites and in-center treatments at its dialysis clinics. Some data is subject in part to extrapolations.
3 Data of Fresenius Helios’s fertility services division include in 2022 only the Spanish entities.

