The safety of our patients is our highest priority, and it plays a central role. In our medical services, for example, disruptions in the process flow, such as natural disasters or technical failure, pose a significant risk to patients and the clinics. In addition, there are also operational risks, for example due to hygiene deficiencies. We counter these risks through structured processes, training, and quality management systems, among other things, and work to continuously improve patient and product safety. Transparent information to for the public is also part of our safety and quality commitment. The potential consequences that these and other non-financial risks can have for Fresenius are described in detail in our Opportunities and Risk Report.
Our approach
At Fresenius, our aspiration is: Ever better medicine for ever more people. In order to provide patients with the best possible care, we offer them medical treatments and products that meet our strict requirements for quality and safety. It is essential for the safety and well-being of our patients that we appropriately label our products, describe our services in a transparent manner, and provide all relevant information to patients or their relatives in our health care facilities. For health care professionals, relevant information on pharmaceutical products or medical equipment is provided through dedicated communication channels, for example websites, and trained experts from our business segments.
We have established sophisticated and efficient processes in all business segments that are fully geared toward the safety of our patients. In the area of quality management, we monitor, manage, and improve these processes with performance indicators. Each individual business segment adapts its quality management system and sets priorities according to its respective business model. Our quality management systems meet and are based on various standards or are adapted to them. International standards such as ISO (International Organization for Standardization) and GMP (Good Manufacturing Practice) are particularly important for our production facilities. Our hospitals and health care facilities measure the quality of patient care using various indicators. Each of the four business segments is subject to specific regulatory requirements and standards, depending on the business activity and the market.
We use different applications to check our quality management systems, depending on the business segment and business activity. We use externally provided IT systems as well as self-developed applications. All units are subject to regular external and internal audits. Peer reviews in hospitals are carried out if the internal quality targets are not met. We report on the evaluation and outcome of audits for each business segment.
Training courses for our employees, which are an essential part of guaranteeing the safety of our patients and products, are an important component of our quality management systems.
By offering regular training on a global, regional, and local level, Fresenius Kabi ensures that employees are aware of all aspects of the quality management system that are relevant for their daily work. For more information on quality management training at Fresenius Kabi, see the Employee development section.
Helios Germany has three simulation and emergency facilities in Erfurt, Krefeld and Hildesheim. Among other things, surgical procedures or crisis scenarios in the operating room are trained here. In addition, such training courses take place in the clinics directly. In the fields of emergency medicine, anesthesia, intensive care medicine, and obstetrics, decisions on the content and number of participants in the mandatory training courses are based on resolutions of the respective specialist groups. Helios Spain continuously provides training on patient safety, on its quality management systems and on topics that are essential in hospital routine. In 2021, 14 sessions or courses were conducted in the hospital network. The exchange of knowledge among the hospital network has been promoted through inter-hospital clinical sessions that now cover several medical fields such as gynecology and obstetrics, pediatrics, and internal medicine. In addition, clinical sessions have been held on several patient safety topics: best patient safety practices in the surgical block, preventing adverse events in the insertion and management of venous access, patient falls prevention, medical record and informed consent completion requirements, transforming the patient safety culture of the hospital through the Joint Commission International accreditation process, and evidence-based safety improvement practices.
Fresenius Vamed’s quality management officers also regularly conduct legally required training courses and quality management training courses. In addition, Fresenius Vamed plans and conducts in-person and online training courses on a wide range of topics.
Further information on employee training can be found in the Employee development section.
Organization and responsibilities
All Fresenius employees must ensure that the applicable quality and safety regulations are always applied in their areas of responsibility. The employees in the production facilities, outpatient centers, and hospitals have a special obligation to exercise due care. The organizational structures are adapted to the requirements of the individual business segments.
Policies and regulations
All four business segments comply with the applicable laws within the framework of quality management. This includes the EU legislation on the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH), the Restriction of Hazardous Substances (RoHS), the Medical Device Regulation (MDR), and the Code of Federal Regulations (CFR) of the U.S. Food and Drug Administration (FDAFDA (U.S. Food & Drug Administration)Official authority for food observation and drug registration in the United States.), among others.
In addition, the business segments have developed their own comprehensive guidelines. Furthermore, they have voluntarily committed to complying with a wide range of industry obligations and international standards.
Certifications and commitment
Our commitment to patients` health and well-being in the business segments is reviewed and certified by external partners or regulatory bodies. We are continuously expanding the number of sites certified to ISO 9001 standard, applicable international acknowledged care or hospital standards or quality standards provided for centers of expertise for certain areas of treatment. Not all locations have the same scope of certifications. However, at the very least they adhere to internal quality standards, which are subject to applicable regulatory provisions.
Quality principles or standards applied in addition to the internationally acknowledged ISO 9001 are, among others,
- the methodology of the Initiative for Quality Medicine (IQM), the model EFQM, the standards of the Joint Commission International (JCI), and the Spanish UNEUNEThe Spanish Association for Standardization, UNE, is the body legally responsible for the development of standards in Spain. It is the Spanish representative in ISO., for health care facilities, and
- Good Manufacturing Practice (GMP), Good Distribution Practice (GDP), Guideline on Good Pharmacovigilance Practices (GVP), Medical Device Regulation (MEDDEV; MDR), the Code of Federal Regulations (CFR) of the U.S. Food and Drug Administration (FDA), and the ISO 13485 quality management standard for medical devices in our production business of Fresenius Medical Care and Fresenius Kabi.
In 2021, further locations were added to ISO 9001. Due to the COVID-19 pandemic, certifications at Helios Spain planned for the 2020 reporting year were started in the first quarter of 2021 and successfully finalized in March 2021.
The Fresenius Group quality management approach is controlled by internal specialists or dedicated functions within the business segments. Relevant data is reviewed regularly, for example daily. If deviations occur, our specialists initiate root cause analyses or peer reviews; they evaluate deviations and, if necessary, determine corrective or preventive actions. Regular internal audits and self-inspections, at least annually, often at higher frequencies, support data verification and management approaches, for certified and non-certified entities. Thus, we ensure that patient health activities comply with internal guidelines and regulatory provisions. The overarching ambition is to improve the efficiency and coverage of our quality management systems and, ultimately, the credibility of the procedures and systems in place.
Following a risk-based approach, Fresenius Medical Care carries out internal audits at least once a year at each of its production sites. The business segment assesses its quality management systems against internal and regulatory standards. Internal quality audits at the local sites help the business segment determine the effectiveness of these systems. The consolidated quality management system is certified according to ISO 9001 and ISO 13485. Fresenius Medical Care also completed the Medical Device Single Audit Program (MDSAP) for this system. The production sites are subject to regular external quality audits and reviews in accordance with local requirements. Audits are carried out according to the Good Manufacturing Practice (GMP), the Current Good Manufacturing Practice (cGMP), ISO 9001, ISO 13485, or MDSAP. In 2021, 74% of the production sites managed by the Global Manufacturing, Quality, and Supply division were certified to ISO 9001 / 13485.
Quality standards Fresenius Medical Care
Download(XLS, 36 KB)| Quality standard1 | ISO 9001/13485 | GMP/cGMP | MDSAP |
|---|---|---|---|
| Production sites certified2 (%) | 74 | 49 | 29 |
| 1 Increased scope in 2021 following acquisition of Xenios plants into the Global Manufacturing, Quality, and Supply division. | |||
| 2 Production sites managed by Global Manufacturing, Quality, and Supply division. | |||
Fresenius Kabi’s quality management system is organized in accordance with the ISO 9001 standard and is binding for all organizations of the business segment. Compliance with the standard is reviewed by TÜV Süd in annual audits at a global level and covers 102 Fresenius Kabi organizations through a matrix certification; further organizations hold local ISO 9001 certificates. In addition, numerous manufacturing plants are also certified according to ISO 13485 for medical devices as well as GMP.
Quality standards Fresenius Kabi
Download(XLS, 35 KB)| Quality standard | ISO 9001 | ISO 13485 | GMP/cGMP |
|---|---|---|---|
| Number of certified entities | 104 | 23 | 34 |
| Number of certified entities1 (%) | 78 | 100 | 100 |
| 1 Coverage target 100% of relevant entities, variation due to organizational changes, e.g., opening, closing of locations; %-coverage subject based on entities for which the standard is of relevance. | |||
Helios Germany applies the German Inpatient Quality Indicator (G-IQI) management system in all German clinics. Newly acquired entities are integrated into this management system from the start of the acquisition. Further certifications encompass the acknowledgment as centers of medical expertise, e. g., for oncology, diabetes, endoprosthetics, or others.
Helios Spain gears its quality management toward the requirements of recognized international quality standards. All hospitals and centers are certified according to ISO Standard 9001 and continued to be certified according to the Spanish Association for Standardization, UNE. New acquisitions conducted in 2021 will be included in the certification in 2022. 33% of the hospitals are additionally certified under the quality standard UNE 179003. In 2021, 4 further hospitals were awarded the international certification UNE 179003, a total of 16 hospitals for the reporting year (2020: 12; 24%). 11 hospitals are also already certified to UNE 179006, the standard for infection control (2020: 8). In addition, we have 12 assisted reproduction units certified with UNE 179007.
In 2021, two further hospitals were included in the JCI certification. In total, five hospitals (including clinics in Latin America) are accredited with JCI and four hospitals with the European Foundation for Quality Management (EFQM) standards. Fundación Jiménez Díaz was the first hospital in the world to receive the EFQM Global award. The hospital has obtained more than 750 points, which also gives it the EFQM 7 Stars seal, the highest score for this standard.
Quality standards Helios Spain
Download(XLS, 35 KB)| Quality standard | ISO 9001 | UNE 179003 | UNE 179006 | UNE 179007 |
|---|---|---|---|---|
| Number of certified locations | 49 | 16 | 11 | 12 |
| Number of certified locations (%)1 | 100 | 33 | 23 | 25 |
| 1 %-coverage subject based on entities for which the standard is of relevance | ||||
Fresenius Vamed aligns its internal processes to established quality standards such as ISO 9001, the sector-specific standard EN15224 for quality management in health care, and ISO 13485, as well as the EFQM standards. In addition, Fresenius Vamed has certified several health care facilities according to international standards such as JCI, ISO, or the German QMS-REHA (BAR). All inpatient rehabilitation facilities in Germany must be certified in accordance with a procedure recognized by the Federal Association for Rehabilitation (Bundesarbeitsgemeinschaft für Rehabilitation e.V. – BAR). All certifications form the basis for the continuous improvement of the processes at Fresenius Vamed.
In Germany, as required by law, the rehabilitation clinics have been accredited in accordance with the requirements of the Federal Association for Rehabilitation (BAR) and certified according to the quality seal of the Private Hospital Association Schleswig-Holstein e.V. (German: Gütesiegel Medizinische Rehabilitation in geprüfter Qualität der Krankenhausgesellschaft Schleswig-Holstein e.V.). One exception is a geriatric clinic, which is certified according to the DIN EN ISO 9001 / Geriatrics quality seal.
All rehabilitation clinics in Austria are certified or are to be certified to at least one standard of ISO, JCI, or QMS-Reha.
Quality standards Fresenius Vamed
Download(XLS, 35 KB)| Quality standard | ISO 9001 | ISO 13485 | JCI or other |
|---|---|---|---|
| Number of certified locations | 31 | 13 | 136 |
| Number of certified locations (%) | 74 | 1001 | 56 |
| 1%-coverage subject based on entities for which the standard is of relevance. | |||
External assessments
Fresenius Kabi USA, e. g., received the Premier Supplier Legacy Award in 2021 for its local customer service, commitment, and value creation through clinical excellence and cost optimization. The award recognized Fresenius Kabi’s efforts in providing vital medicines and technologies in 2020. For more than three years, Legacy Award winners have been contracted suppliers to Premier, a health care improvement company that unites an alliance of approximately 4,100 U.S. hospitals and health systems and more than 200,000 other providers and organizations.
Evaluation
GRI 416/103-3, GRI 417/103-3, GRI 417-1
With regard to patient health and safety in all business segments, breaches or violations that lead to deviations from internal management provisions have to be evaluated. Resulting corrective and preventive actions aim to ensure our patients’ health and safety. Information on the identification of deviations and examples of possible deviations can be found in the following reporting on the business segments.
Fresenius Medical Care
Patient well-being is top priority. As part of the business segment’s commitment to delivering safe, high-quality care to patients with chronic illnesses, it continually monitors the performance of its products and services. The focus is on quality, safety, accessibility, and patient experience. Fresenius Medical Care makes further improvements where necessary, keeping in mind the goal to expand access to high-quality health care. The business segment invests in innovations and new technologies, and leverage insights from scientific research and collaboration with partners.
Fresenius Medical Care develops, produces and delivers a broad range of products for treating kidney disease. With its network of production sites around the world, the company controls the procurement, production, distribution, and supply of renal and multi-organ therapy products. The business segment manages quality and safety in its product business over the entire product life cycle, from design and development, to operation and application.
Organization and responsibilities
The Global Medical Office drives the medical strategy and coordinates activities related to the advancement of medical science and patient care. It is part of the business segment’s network that promotes scientific and medical progress worldwide. The Global Medical Office is led by the Global Chief Medical Officer who is also a member of the Management Board of Fresenius Medical Care. Key findings of the Global Medical Office are reviewed by dedicated committees. They are published on a regular basis and shared with the medical community.
The Global Research and Development and the Global Manufacturing, Quality, and Supply divisions are responsible for the product business of Fresenius Medical Care. The functions report directly to the Management Board of the company.
Internal rules of conduct and guidelines
Fresenius Medical Care’s commitment to continuously improve the quality of care is included in their Code of Ethics and Business Conduct. The Global Patient Care Policy outlines the principles, responsibilities, and processes related to patient experience surveys and grievance mechanisms. In 2021, a chapter on medical strategy and quality management was included in this policy. Responsibility for integrating the policy into operations lies with senior medical leadership and the interdisciplinary patient care teams in each of the regions.
The Global Quality Policy outlines the company’s commitment to product and service quality. The policy also covers the obligation to comply with relevant regulations, and maintain environmentally sound and efficient operations. It is the basis for regional quality manuals and further policies covering responsibilities, training, risk assessments, and audits. The Management Board is regularly informed about the global quality performance.
Over the past few years, Fresenius Medical Care has merged the quality management systems in Europe, Middle East, and Africa, Latin America, and Asia-Pacific.
Patient information
Fresenius Medical Care treats patients across the full spectrum of chronic kidney disease. The company believes listening to their therapy preferences is critical. The company aims to give patients an informed choice and provide treatment options that best fit their circumstances. Home dialysisDialysisForm of renal replacement therapy where a semipermeable membrane – in peritoneal dialysis the peritoneum of the patient, in hemo dialysis the membrane of the dialyzer – is used to clean a patient’s blood. provides patients with the opportunity for greater independence and control over their time and health outcomes.
Our ambitions
Fresenius Medical Care has set itself the goal of implementing a global quality management system by 2024. Additionally, the IT tool for audit management has already been harmonized globally, and the company plans to introduce a global electronic training system by 2024.
Fresenius Medical Care has defined its first global KPI for quality of care – the global hospitalization rate. It measures the length of time a patient spends in hospital.
Fresenius Medical Care is also planning to develop a quality index focusing on the most relevant quality indicators to reflect improvements and achievements related to global patient care.
Having recently achieved the internal NPS target, the business segment is now aiming for a NPS score of at least 70.
Progress and measures 2021
Fresenius Medical Care works with external organizations to facilitate scientific progress and explore new ways of improving quality of care. In 2021, the business segment was involved in more than 60 key partnerships with academia, research institutes, and peers. Focus areas included cardio-protection, personalized and precise medicine, public health, and the impact of COVID-19 on vulnerable patient populations.
Evaluation
During the COVID-19 pandemic, Fresenius Medical Care has worked to keep the clinical care environment as stable as possible and deliver a high quality of care. Further information is provided on the company's website, see the Sustainability section.
Quality analyses
Fresenius Medical Care continually measures and assesses the quality of care provided in its dialysis clinics based on generally recognized quality standards and international guidelines. These include those of the global nonprofit Kidney Disease: Improving Global Outcomes, the Kidney Disease Outcomes Quality Initiative, and European Renal Best Practice. The business segment also considers industry-specific clinical benchmarks and its own quality targets.
Additionally, Fresenius Medical Care evaluates a set of medical indicators on an ongoing basis to measure the quality of care provided in its dialysis clinics. The global hospitalization rate measures the length of time a patient spends in hospital. In 2021, the global hospitalization rate was 10.7 days per patient. This is an important indicator, given hospitalization has a significant impact on a patient’s quality of life. It also reflects the business segment’s impact on the respective health care system, which is especially relevant during the ongoing pandemic. Other quality of care KPIs are currently measured on a regional level as Fresenius Medical Care continues to harmonize these criteria.
Patient satisfaction
As part of the global patient experience program, Fresenius Medical Care aims to conduct patient experience surveys at least every two years. The business segment uses the information collected to evaluate the services provided by its dialysis clinics and implement global improvement processes. Fresenius Medical Care’s goal is to establish measures that enable more personalized care and improve the quality of services. Based on the results of the 2020 survey, in 2021 the business segment sharpened its focus on improving patient education, individualized patient care, and service excellence. For example, the business segment developed patient education material to help clinic staff better inform their patients about health-related topics.
Fresenius Medical Care measures patient experience and customer loyalty using the Net Promoter Score (NPS). The NPS reflects patients’ overall satisfaction with the services. In 2021, the NPS was 71, compared with 67 in 2020. The increase can be attributed to comprehensive local improvement measures, such as those mentioned in the paragraph above. In line with the mission to provide a future worth living for the patients, the business segment is continuously working toward improving patients' experience. As part of the NPS calculations, the percentage of patients is measured that would recommend Fresenius Medical Care. In the reporting year, 78% of the patients answered in the survey that they would highly recommend the services.
In addition to the NPS, Fresenius Medical Care also tracks survey coverage and response rates. In 2021, a global coverage rate of 91% was achieved, in line with the target of 75% or above. In 2021, the response rate was 75%.
Fresenius Medical Care Net Promoter Score
Download(XLS, 36 KB)| Year | 20211 | 2020 |
|---|---|---|
| NPS2 | 71 | 67 |
| Coverage rate3 (%) | 91 | 78 |
| Response rate4 (%) | 75 | 76 |
| 1 Figures are based on the latest data from patient surveys rolled-out in Fresenius Medical Care dialysis clinics. In some cases, this is data from 2020 because surveys are only conducted bi-annually in some regions. | ||
| 2 The NPS is a value between -100 and 100. | ||
| 3 The coverage rate reflects the percentage of eligible patients that were invited to participate in the patient experience survey. | ||
| 4 The response rate reflects the percentage of eligible patients that answered the survey (including the question relating to the NPS). | ||
Patient grievance processes
In addition to the experience survey, Fresenius Medical Care provides patients and their representatives with other feedback channels. They can use these to make any suggestions or raise concerns, anonymously if they wish. Channels include dedicated hotlines and email addresses, complaint and suggestion boxes, and a feedback form on the company website. The business segment is committed to resolving any issues in a timely manner.
In the reporting year, the company received 24,449 patient reports through local feedback channels.
The business segment's policies allow patients to report grievances without fear of reprisal or denial of services. In most regions, concerns that are dealt with on the spot are not considered grievances. Fresenius Medical Care provides training to support staff in following patient grievance guidelines.
Handling product complaints
Post-market surveillance is an integral part of quality management. It is essential that products and services are effective and reliable, and pose as low a risk as possible to patients. Standards for planning, conducting, and monitoring clinical studies help to enhance product quality and safety and improve patients’ health. Should any issue arise concerning the safety of our products, Fresenius Medical Care takes corrective action. This could include publishing further information and data on the product after market introduction, or product recall.
Fresenius Medical Care strives to comply with legal and regulatory requirements in monitoring the adverse effects of drugs – also called pharmacovigilance – and medical devices. The business segment collects and reviews adverse events and product complaints. The company has incorporated the topic of reporting adverse events and product complaints in its Code of Ethics and Business Conduct.
Audits
Fresenius Medical Care has defined key performance indicators to monitor its quality objectives and prevent adverse events. The company discloses the audit score, which indicates the ratio of major and critical findings to the number of external audits. In 2021, more than 50 certification audits were performed at productions sites that are managed by the Global Manufacturing, Quality, and Supply division. The audit score was 0.1 (2020: 0.2). Fresenius Medical Care targets an average global audit score not exceeding 1.0 to maintain the effectiveness of its quality management systems and certifications. All audit findings are documented and escalated depending on their criticality and are used to determine and implement appropriate corrective and preventive measures.
Fresenius Kabi
Fresenius Kabi’s corporate philosophy “caring for life” describes the company’s commitment to improving the quality of life of its patients. The quality and safety of its products and services is therefore of paramount importance to the business segment. An important goal of the quality management at Fresenius Kabi is to monitor the applicability, efficacy, and safety of products and services, as well as the success of therapies, and their continuous improvement. To ensure this, the company has established an integrated quality management system, a monitoring and reporting system, and product risk management.
Fresenius Kabi has global standard operating procedures as well as a quality management manual that includes, among other things, the company's quality policy, which also applies to all sites.
The business segment uses a global electronic quality management system, KabiTrack, based on the Trackwise® software, for event and change control of quality management processes. The system supports the local implementation of centrally defined processes as well as global oversight.
integrated quality management fresenius kabi
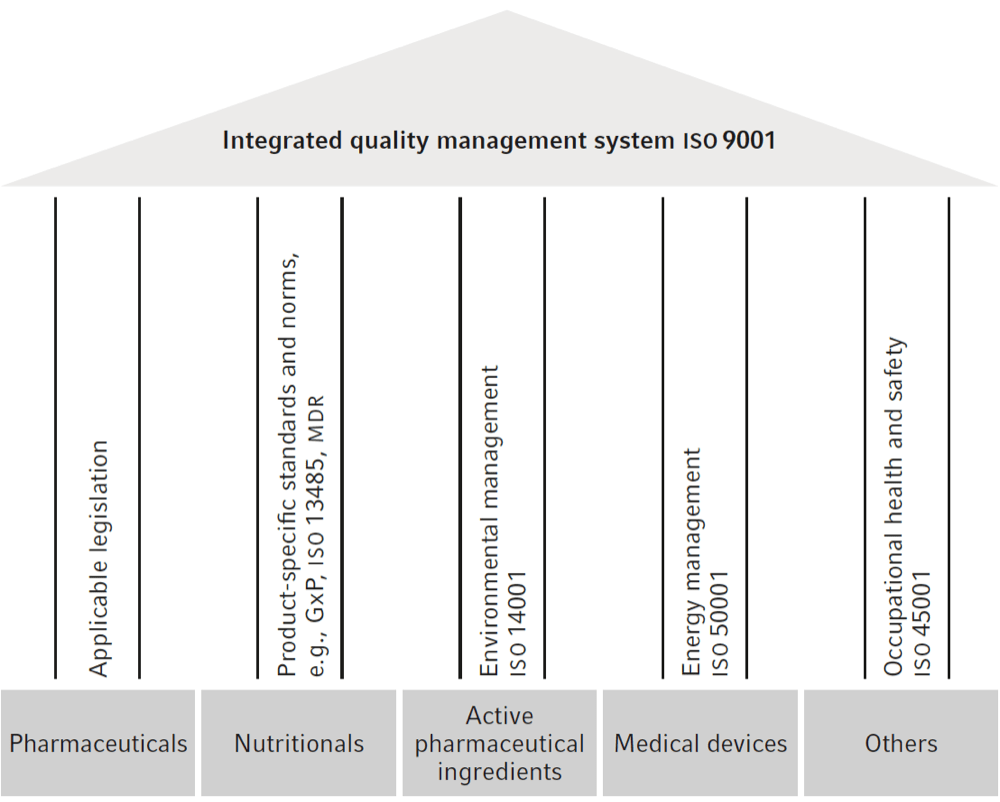
Organization and responsibilities
At Fresenius Kabi, the globally responsible quality managers report directly to the respective member of the Management Board. The members of the Management Board are directly responsible for quality management. They attend quality oversight meetings and receive quality reports on a regular basis.
Policies and regulations
Fresenius Kabi has defined the following principles for its quality management:
- Clear assignment of responsibilities
- Qualification and continuous training of employees
- Transparent and documented processes and procedures
- Fulfillment of regulatory compliance
- Continuous improvement
- Checking of quality management effectiveness
Monitoring and reporting systems
Fresenius Kabi interacts with patients, users, and customers in the provision of products and services, and monitors the applicability, effectiveness and safety of its products on the market. Further, the business segment monitors and evaluates relevant information and feedback on the products, services, and therapies during their use. Fresenius Kabi has set up monitoring and reporting systems, e. g. a vigilance system, and a product risk management system covering all regions worldwide, in order to be informed and deal with product quality and patient safety issues in a timely manner. These early-warning systems are designed in such a way that trained complaints and safety officers worldwide record complaints and side effects in IT systems and forward the respective information to experts for review.
Product risk management
Global safety officers react promptly and appropriately to potential quality-related issues. They initiate and coordinate necessary actions, such as product recalls, on a global level. With its early-warning system, Fresenius Kabi evaluates any quality-related information from various risk areas to identify risks early and take corrective and preventive actions. Information is obtained from databases for complaints and side effects, internal and external audits, and from key performance indicators used for internal control and optimization of quality processes. With these systems, Fresenius Kabi can evaluate the safety profile of any of its products at a global level continuously.
Product recalls, for example, are initiated as a risk-minimizing measure in cooperation with the responsible regulatory authority. At the same time, the cause of the recall is analyzed.
Where necessary, corrective measures are taken to prevent the cause of the recall in the future.
Labeling and product information
Fresenius Kabi’s products are classified, e.g., as pharmaceuticals, nutritional products, active pharmaceutical ingredients, or medical devices, based on global and national regulations and standards. The marketing of these products is subject to various laws and regulations to ensure complete and fact-based product information. Fresenius Kabi has a global policy and global standard operating procedures for its product information to ensure that it is in accordance with applicable laws and regulations and that the product information is correct, accurate, and not misleading.
The products of Fresenius Kabi are also subject to certain labeling requirements. The labeling of the products is checked as part of the regular pharmacovigilance activities – e. g., compliance with laws relating to side effects of medicinal products – and updated if necessary. For example, product labeling is updated if competent authorities, e. g., the Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA), publish relevant information. The dedicated function at Fresenius Kabi uses an electronic management system for product labeling or any printed packaging material to manage the information necessary for labeling and to ensure correctness. The requirements of the European Falsified Medicines Directive or the U.S. Drug Supply Chain Security Act (DSCSA) lead the way in this context. Fresenius Kabi takes into account their specifications and has introduced appropriate processes for serialization, testing, and traceability for the relevant products. Further information on transparency in health care can be found in the section Compliance.
With the help of its vigilance activities, Fresenius Kabi ensures that the patients’ safety of its products is always guaranteed: In this way, the company can identify any changes in the benefit risk ratio of its products at an early stage and reacts in a timely manner. Fresenius Kabi’s Corporate Safety Officer is responsible for the global vigilance system. This function ensures that the company can respond quickly to safety-relevant events. Fresenius Kabi promptly informs its customers and the public about matters concerning product and patient safety; this may be done directly or through appropriate public relations, if applicable.
The new requirements for medical devices as a result of the Medical Device Directive (MDR) issued by the EU in 2017 came into force on May 26, 2021. Thus, the focus on patient safety was significantly tightened for all medical devices on the European market, including the requirements for respective vigilance systems. Fresenius Kabi has adapted its processes in accordance with the new regulation. For example, Fresenius Kabi integrated the shortened reporting timelines to the responsible authorities into its internal processes.
Our ambitions
At Fresenius Kabi, the application of the highest possible quality and safety standards, the efficacy of products and services, and the adherence to regulatory assessment and compliance requirements, are essential conditions to support the business segment’s goal: to ensure its long-term success. Fresenius Kabi continuously promotes a quality and safety culture and aims to ensure compliance with increasing regulatory requirements and expectations of regulatory bodies in an effective manner.
Progress and measures 2021
In the reporting year, the management approach and governance structure of Fresenius Kabi remained as reported in 2020. Progress focused on implementing the requirements of the EU Medical Devices Directive (MDR), which has been in force since 2021.
Evaluation
Fresenius Kabi assesses the health and safety impacts of all significant product and service categories. Further, Fresenius Kabi aims to assess products for improvement potential. Further information is provided in the Research & development section in Group Management Report.
Vigilance system
The monitoring of adverse reactions or events (side effects) associated with the use of medicinal products is referred to as pharmacovigilance (drug safety). The statutory pharmacovigilance commitments relate to our medicinal products for human use. Similar regulations exist for medical devices. Fresenius Kabi has established various standard operating procedures for the continuous monitoring of the benefit-risk ratio of its own products and assesses their successful implementation based on specific indicators.
- Fresenius Kabi collects and assesses reports about individual side effects and reports them to health authorities worldwide in accordance with regulatory requirements. The business segment claims to submit all safety reports in accordance with the applicable regulations and therefore strives to report 100% of the Individualized Case Safety Reports (ICSRs) to the authorities in time. For 2021, the worldwide compliance rate was 99.6% (2020: 99.9%). In Europe, in 2021 99.6% (2020: 99.5%) of all adverse reactions were reported to the European Medicines Agency (EMA) in due time.
- In addition, Fresenius Kabi regularly evaluates the benefit risk ratio of its products based on safety-related information from various sources (e. g., adverse event reports, medical literature). The results of these analyses are submitted to authorities as periodic safety reports. Fresenius Kabi aims to submit all periodic safety reports worldwide to authorities in due time. For 2021, the compliance rate was 98.9% (2020: 99.6%). In Europe, 98.8% of all periodic safety reports were submitted in due time to the EMA in 2021 (2020: 98.6%).
- According to regulatory requirements, Fresenius Kabi, as a pharmaceutical company, is obliged to describe its vigilance system in a Pharmacovigilance System Master File (PSMF). Fresenius Kabi uses a global database to collect and evaluate vigilance data on a quarterly basis from all local marketing and sales units for the PSMF. The goal is to receive timely data from all marketing and sales units worldwide. This is documented in the company’s vigilance system. For 2021, the compliance rate was 100% (2020: 100%).
In addition to the timely evaluation and reporting of single side effects to authorities, cumulative evaluations on side effects are carried out to guarantee the safety of our products (signal detection). These include important events, e. g., reports about side effects with a fatal outcome to evaluate if new information is available about a known side effect profile or a new side effect of a product leading to a changed risk profile. No such information became known in the reporting year about side effects of the business segment’s products.
Audits and inspections
Fresenius Kabi regularly conducts internal quality audits to ensure the effectiveness of the quality management system and compliance with internal and external standards and requirements. The suppliers related to product manufacturing are subject to a qualification process based on the relevance of the delivered material or service. Also, the supplier’s qualification and their recertification is regularly audited. Inspections by regulatory authorities and audits by independent organizations and customers are performed along the entire value chain at Fresenius Kabi. Fresenius Kabi promptly takes steps to deal with any weaknesses or deficiencies discovered during inspections.
The external audits and inspections in the reporting year comprised a total of 30 inspections (2020: 21) regarding Good Manufacturing Practices (GMP) by the U.S. Food and Drug Administration (FDA), the Australian Therapeutic Goods Administration (TGA), Canada Health, European regulatory authorities, and Quality System audits from TÜV Süd (notifying body for ISO 9001).
Audits and inspections
Download(XLS, 35 KB)| 2021 | 2020 | 2019 | |
|---|---|---|---|
| Internal audits | 58 | 42 | 60 |
| External audits and inspections | 94 | 59 | 64 |
Based on the respective observations, an audit and inspection score is calculated. The score is calculated by addition of the number of critical and major observations identified during GMP inspections by the authorities mentioned above and the number of non-conformities identified during TÜV Süd ISO 9001 audits, divided by the overall number of inspections and audits; critical observations, if any, or certification status withdrawal are weighted with a multiplier compared to major observations. The audit and inspection score was 1.9 in 2021 (2020: 1.3)1. Observations have been and will be addressed by corrective and preventive actions (CAPAs) and effectiveness checks have been and will be defined. The observations neither impacted the GMP certification nor the ISO 9001 certificate.
In 2021, no events with a material adverse impact were recorded that conflict with our quality management goals.
1 For the calculation of the audit and inspection score, Fresenius Kabi takes into account all information on findings from audits and inspections received by the company before December 31, 2021.
Fresenius Helios
Helios Germany has developed a quality management system based on three pillars: Measure, Publish, Improve, used by around 500 hospitals in Germany and Switzerland. This quality management system is based on administrative data (routine data) from patient treatments: the hospitals document each treatment step for later billing with the health insurance companies.
This routine data shows whether the healing process took longer than expected, and whether complications or even a death occurred. It also indicates whether a treatment took a normal course; if mistakes were made, they are reviewed in specific audit procedures (peer reviews). Defined quality indicators (German Inpatient Quality Indicators – G-IQI) are used to measure and monitor the quality of medical outcomes, which are published. This data allows patients to see, among other things, how often certain treatments are performed in Fresenius Helios hospitals. It also gives patients important information on the doctors’ experience and routine and helps inform their own decisions about their treatment. Thanks to its quality and risk management, Helios Germany can continuously monitor key quality parameters and, if necessary, take countermeasures at an early stage.
In Spain, Fresenius Helios implemented the IQI methodology at the end of 2017 in all its hospitals. Since then, 45 indicators have been systematically monitored, first on a quarterly basis, and since 2021 on a monthly basis.
Organization and responsibilities
At Fresenius Helios, the medical director has direct responsibility for patient and product safety and a Patient Safety Officer position has also been created. Helios Germany’s central medical services and medical specialist groups help to implement appropriate measures. The leading physicians in various fields from all Fresenius Helios hospitals in Germany come together to form specialist groups. They ensure that the knowledge of their medical specialty is anchored in all hospitals and represent their respective medical fields internally and externally. They also advise and decide on the introduction of standard processes, sensible innovations, campaigns, and the introduction of medical products.
In Spain, the organization has been reinforced with the creation of a new Corporate Operations department, focused on improvements in the provision of therapies and health services and the design and marketing of new digital products in the ambulatory setting. The Corporate Risk unit has also been created in order to improve risk management in the company.
Helios Germany and Helios Spain’s specialist groups exchange ideas and information on specific topics. For example, the German hospitals benefit from Helios Spain’s close networking of outpatient and inpatient care – and can take advantage of these experiences.
Policies and regulations
Helios Germany has built on the numerous measures introduced in the past, to increase patient safety. Two checklists are mandatory for all surgeries in Fresenius Helios’ clinics. The “PRÄ” checklist assesses the risks associated with the surgery before it takes place. The second checklist, “PERI”, helps to avoid treatment errors immediately before, during, and immediately after the surgical procedure.
Since 2020, these measures have been supplemented by increased hygiene requirements due to the COVID-19 pandemic. For this purpose, the existing hygiene concepts have been adapted to the changed regulatory provisions.
In 2021, the Corporate Patient Safety Committee at Helios Spain continued to develop and implement clinical best practices. The committee consists of members from various hospitals, including clinics in Latin America. The committee has remained active throughout the pandemic, not only advising the hospitals on COVID-19 management, but also developing new strategic patient safety protocols and updating them in 2021. The existing Patient Safety Strategy developed by this committee will be updated in 2022. It is based on principles such as those of the World Health Organization (WHO) and the JCI.
Hygiene management in hospitals
The aim of Fresenius Helios’ hygiene management system is to avoid infections within the hospital and to quickly prevent them from spreading when they do occur. Hygiene management focuses on close monitoring of infections and pathogens, regular hygiene training for hospital staff, for example on correct hand disinfection, monitoring antibiotic consumption, and training physicians as antibiotic stewardship (ABS) specialists. The implementation of and compliance with hospital hygiene measures in the clinics is accompanied and monitored by our specially trained staff – hygiene specialist nurses, hospital hygienists and hygiene officers. The Helios Group hygiene regulation is binding for all employees in all clinics. It is based on the evidence-based recommendations of the Robert Koch Institute (RKI) and prescribes, among other things, hand disinfection – especially before and after contact with patients – for physicians, nurses, medical staff, and other personnel, in accordance with the guidelines of the World Health Organization (WHO).
The clinics monitor their hygiene status continuously and transparently: every six months, Fresenius Helios publishes figures for each clinic on the occurrence of the three most important multi-resistant and infection-relevant pathogens. The reporting for 2021 is delayed to the first half of 2022 due to the impacts caused by COVID-19.
In Spain, Fresenius Helios conducts training courses on hygiene management on a regular basis. The clinical group participates in the EPINE program – a monitoring system for nosocomial infections that occur during or after hospital treatment. The program is coordinated by the Spanish society of preventive medicine, public health, and hygiene, and supported by the Spanish Ministry of Health and the European Center for Disease Prevention and Control. Local data is collected from the infection services of the participating hospitals with the aim of improving the quality of care in the hospitals.
In 2020, Helios Spain started encouraging all hospitals to appoint a medical officer to manage infection control and prevention measures. In 2021, 50% of the hospitals had an epidemiologist to manage infection prevention and control within the hospital. Other hospitals had at least one professional specialized in infection control from other specialties (internal medicine, intensive care) who manages this area.
As part of its quality management system, the business segment monitors a bundle of indicators related to hand hygiene compliance and to the prevention of nosocomial infections, such as the WHO Hand Hygiene Self-Assessment Framework and a central-line-catheter-related bacterial infection rate.
Patient information
Fresenius Helios provides information to its patients within its hospitals about the patient admission process with the help of the treatment contract, as well as special information documents and privacy statements. The therapeutic objective is discussed with patients during admission and discharge discussions with the treating physicians. Fresenius Helios communicates via an online magazine, social media, its website, and in its communication campaigns for the interested public.
In addition, information events on specific medical topics are held in all hospitals (known as patient academies). Further details on transparency in health care can be found in the Compliance section.
Our ambitions
Helios sets company goals to measure the quality of treatment in its hospitals, using the E-IQI methodology in Spain and the G-IQI methodology in Germany. Each hospital treatment (case) is evaluated by making use of comparative measurements, with the benchmark being the German national average as calculated by the Federal Statistical Office or comparable national benchmarks in Spain. The target is in each case to be better than the national average for the respective indication. Further quality targets in our hospitals in Spain relate to patient satisfaction and are measured via the NPS, among other methods.
Progress and measures 2021
In the reporting year, the management approach and the governance structure of Fresenius Helios remained mainly as reported in 2020.
The IQI methodology will be extended to the clinics in Latin America. In addition to the implemention of the necessary medical and patient data base, the medical documentation will be improved to gather the data relevant to calculate the IQI development.
As an additional tool for improving patient safety, the Corporate Committee on medical liability claims has been reorganized so that medical directors of the hospitals can now participate, in order to improve the healthcare risk management in the hospital network.
A specific policy to enhance the early diagnosis of sepsis was launched in 2021 at our Spanish hospitals. Also, to assist in its deployment, two training videos have been developed using real cases of patients with sepsis, to reinforce the key points that have to be taken into account in order to quickly identify this time-dependent pathology.
Evaluation
Fresenius Helios assesses the health and safety impacts of all significant treatment or service categories for improvement potential.
In order to ensure that all physicians working at a hospital in Spain perform clinical acts for which they have demonstrated competence, a model has been defined to validate these competencies and accredit the professional to perform the corresponding acts and procedures. This model has been included in a corporate policy that also defines the monitoring of physicians´ complication rates through the minimum basic dataset (MBDS).
Evaluation of the quality of outcomes
For Fresenius Helios, the quality of medical outcomes is key. Helios Germany has defined specific targets for 47 (2020: 45) key quality indicators, including, for example, the frequency of interventions and their results. Helios Germany’s results are expected to be better than the German average. The year 2021 was as the previous year exceptional for hospitals and was dominated for long periods by the pandemic and the treatment of COVID-19 patients.
In Germany, Helios achieved a total of 43 of its 47 Group targets in 2021. This corresponds to a quality target achievement of 91% (2020: 89%). In 2021, there were around 17% less patients in the clinics than in the pre-COVID-19 year 2019. Many patients stayed at home at the beginning of 2021, because of the nationwide restrictions and the cancellation of surgeries in hospitals in spring. In the second quarter, the number of patients started to increase again at our German locations.
Helios Spain has introduced quality indicators that correspond to Germany’s G-IQI. The results are also compared with the goals of the IQM network. Each hospital publishes its results quarterly, and since 2021 monthly, in a central IT system. This allows individual hospitals to check whether they deviate from the standards set.
The competence gained through the research into COVID-19 and the improved diagnostics related to the infection also led to an improvement in the overall treatment in our Spanish hospitals. Earlier diagnosis, better knowledge of its management and the impact of vaccination, have resulted in less virulence of the illness, less use of intensive care beds and a lower mortality rate. Thus, throughout 2021, the mortality rate of those hospitalized by COVID-19 in Spain has fallen to 11% compared to 15% in 2020, which reached 17% in the first wave in spring 2020.
Helios Germany uses reporting and learning systems for critical events and near-misses of patients in all hospitals (Critical Incident Reporting System – CIRS). In 2021, a total of 576 events were reported in Germany (2020: 458), which were evaluated at the respective clinic level. The central consolidation and analysis of the CIRS data was further rolled out in 2021. In this way, risks relevant for the overall business segment are identified and remediation measures implemented.
At Helios Spain the clinics report patient safety incidents including near misses. In 2021, a total of 8,480 incidents were reported in 2021 (2020: 4,897). At Helios Spain we actively encourage the reporting of incidents, including hazardous (or “unsafe”) conditions and near misses, as a way of promoting patient safety.
Helios Quality Indicators
Download(XLS, 35 KB)| Germany | 2021 | 2020 | 2019 | 2018 | 2017 |
|---|---|---|---|---|---|
| Key indicators, total | > 1,500 | > 1,500 | > 1,500 | > 1,500 | > 1,500 |
| G-IQI-targets | 47 | 45 | 46 | 46 | 45 |
| Targets achieved | 91% | 89% | 96% | 89% | 98% |
| Peer reviews | 7 | 8 | 60 | 55 | 69 |
| Further information (German language only):https://www.helios-gesundheit.de/qualitaet/ | |||||
Helios Germany has its own system for regularly measuring patient safety at its hospitals. This enables deficiencies in patient care to be analyzed and subsequently remedied. The system combines the internationally established Patient Safety Indicators (PSI) with Helios' own indicators. The latter include, for example, the number of abdominal towels accidentally left in the body after an operation, but also side confusion during operations, misdiagnoses, serious medication errors, or falls in the clinic. A company-wide policy for dealing with cases of damage obliges all clinics to systematically record and centrally report these indicators. In 2021, 85 events were recorded for eight selected patient safety indicators (2020: 105).
Helios Germany thus also complies with the recommendations for action newly published by the Patient Safety Action Alliance in September 2021. The central importance of reporting such events is also highlighted by the new Global Action Plan for Patient Safety 2021 – 2030 of the WHO; its purpose is to eliminate preventable harm in healthcare.
Part of Helios Germany's error management is the recording of allegations of treatment errors. At 7681, the number of allegations in the acute clinics in 2021 was lower than in the previous year (2020: 958). In 2021, an average of 0.8 per 1,000 (2020: 0.9) patients made an allegation of a treatment error (justified or unjustified) against Helios hospitals. These allegations include, to varying degrees, all specialties and all stages of treatment, from patient information, diagnostics, surgery, and therapy to aftercare. The goal is to ensure that there is no more than one allegation of treatment error per 1,000 full inpatient treatments.
In the interests of transparent error management, Fresenius Helios handles and settles its liability cases itself as far as possible instead of handing them over to an insurer. As a result, we analyze these cases intensively and learn from them. In addition, Helios Germany developed a tool for use in 2021 that automatically queries preventive measures, which, in the event of a confirmed treatment error, will initiate a central review of the usefulness of the respective preventive measures.
Helios Spain uses an online reporting system for all types of incidents, from near misses to sentinel events. Based on the definition from JCI, a sentinel event is a patient safety event that results in death, permanent harm, or severe temporary harm. The system is accessible for all health care professionals and hospital employees. The reported events are analyzed at least quarterly by each hospital Patient Safety Commission. Trends and causes are identified in order to implement the necessary improvements. This analysis is also recorded in the reporting system and feedback is provided to the notifier.
2 Claims of the acquisition clinics (Malteser, DRK Kassel) from previous years are not fully recorded.
Patient satisfaction measurement and grievance processes
The business segment uses the Helios Service Monitor to measure the satisfaction of inpatients in its German hospital locations once a week. Employees conduct short interviews on care and service. The information is collected anonymously. The management of the hospital and other authorized persons receive the monthly survey results. This makes it possible for necessary improvements to be introduced quickly. In addition, Helios Germany publishes the results of patient surveys, further data on medical treatment quality, and hygiene figures on its corporate website www.helios-gesundheit.de, see Qualität bei Helios (German language only).
In 2021, 713,382 patients nationwide were asked for their personal opinion using the Service Monitor. In this way, the business segment reached around 70% of its inpatients. Of those surveyed, 96 % expressed satisfaction with their current hospital stay.
In Spain, Fresenius Helios uses the net promoter score (NPS) to get specific feedback from patients who have been treated as inpatients, outpatients, or in emergencies. 48 hours after a hospital stay, an e-mail is sent to patients asking if they would recommend the hospital and its services.
The results are analyzed centrally for Helios Spain and at a hospital level by type of treatment and treatment area. The goal is to continuously improve the NPS results. The global NPS score has increased over recent years until the start of the pandemic.
Net promoter score (NPS) Spain
Download(XLS, 35 KB)| 2021 | 2020 | 2019 | |
|---|---|---|---|
| Global NPS | 49.9 | 54.1 | 54.6 |
| Total reports | 534,930 | 361,800 | 426,061 |
In 2021, Helios Spain identified high demand for outpatient consultation that is heavily penalizing NPS results, as the demand from patients is higher than the capacity for consultations and number of doctors in our clinics. The patients expressed their dissatisfaction through low results.
The constraints were increased through the lack of professional staff, impacting the availability of services.
Further information can be found in the chapter Employees in the section Evaluation of this report.
Peer reviews
Helios Germany analyzes the cases – including treatments and medical routines – in hospitals that fail to meet individual quality targets, in order to identify and implement improvements.
Particularly important are the specific audit procedures in the medical and nursing sectors, and the peer reviews – expert discussions of cases. In Germany, specially trained physicians from the hospitals of Helios Germany and from the IQM network cooperate in the peer review, and question statistical abnormalities. Their insights are translated into concrete recommendations for action in the hospital with the aim of increasing patient safety. In 2021, Helios Germany conducted a total of 7 peer reviews (2020: 8), due to the impact of the COVID-19 pandemic and the resulting restrictions on hospital operations.
Due to the pandemic, Helios Spain was able to perform only 4 peer reviews online by the end of 2021 (2020: 2). Internally, ISO 9001 audits were conducted at all Helios clinics in Spain.
Fresenius Vamed
In post-acute care, elderly care and project management, all processes are regularly checked for their suitability and adapted, if necessary. In accordance with the Federal Association for Rehabilitation (BAR) guidelines, Fresenius Vamed implements all relevant measures to increase patient safety at its post-acute care facilities – including patient surveys, complaint management, and regular internal audits of all segments. The company receives feedback on the quality of the structure, process, and outcomes from the insurers, e. g., as part of the quality assurance of the German pension insurance or the statutory health insurance providers. In all Fresenius Vamed health care facilities, patients receive relevant information material and patient training to ensure long-term treatment success. Reporting systems for complaints are also available in some health care facilities. In Fresenius Vamed’s project business, the lead companies establish guidelines for all subsidiaries, which are reviewed in annual audits.
10 fully inpatient facilities at 6 locations provide care for people in need of care in care grades 1 to 5. The range of care and support includes basic care and medical treatment care, social care, day-structuring measures, and additional care for people with a considerable need for general supervision and care (dementia patients), as well as specialized care for people with severe neurological illnesses, with psychiatric or geriatric psychiatric illnesses, and for people with addictive disorders. In addition to full inpatient long-term care, all nursing facilities also offer short-term and respite care.
The nursing facilities are closely connected with the Helios hospitals located nearby so that they can provide residents with the best medical care quickly.
In geriatrics and geriatric care for the elderly and in palliative medicine, Fresenius Vamed has adopted the renowned 'salutogenesis' methodological concept. Leading a self-determined life with dignity! The approach is based on a clear understanding of individual processes to support and maintain the health and well-being of our residents. In nursing and care, we focus on quality of life and a feeling of security and belonging.
Organization and responsibilities
In order to raise awareness of quality requirements among employees, Fresenius Vamed employs staff for quality and risk management. These employees report directly to management.
Quality assurance officers carry out training courses in the various segments, thus integrating all employees in the quality management systems of their facilities. The quality assurance officers can thus ensure that employees comply with their obligation to exercise due care. Fresenius Vamed informs its employees about its understanding of quality early in the initial training and introductory events. Guidelines are communicated to and documented for the relevant areas and departments in writing (e. g. via work instructions from the respective management).
The VAMED International Medical Board (IMB) ensures the exchange of information between Fresenius Vamed physicians from Austria, Germany, the Czech Republic, Switzerland and the United Arab Emirates. Within Fresenius Vamed, medical specialist groups and executive conferences coordinate on quality and safety.
Policies and regulations
Fresenius Vamed sets ethical standards through its mission statement as well as through its Code of Conduct, the Clinical Code of Conduct, and the Code of Conduct for Business Partners.
Fresenius Vamed’s internal guidelines are based on regulatory requirements established throughout Europe, e.g. for rehabilitation. In elderly care, Fresenius Vamed follows the renowned salutogenesis methodological concept. In addition to the statutory requirements and the requirements of the insurers, Fresenius Vamed also adheres to international standards such as ISO and EFQM, expert standards, and medical guidelines. All internal guidelines are regularly reviewed and updated as necessary. Employees can obtain information on the guidelines via the intranet.
Hygiene management in rehabilitation and nursing care
One of Fresenius Vamed’s tasks with regard to hygiene in rehabilitation clinics and nursing facilities is to ensure the highest possible protection for everyone – without restricting individual rehabilitation. Protecting patients from infectious diseases during their stay is a top priority.
Newly established health care facilities follow systematic guidelines from day one to prevent infections breaking out or spreading. Clearly defined procedures are followed and compliance with hygiene regulations is strictly controlled.
Fresenius Vamed’s hygiene standards in Germany are based on the recommendations of the RKI’s KRINKO (Commission for Hospital Hygiene and Infection Prevention). These recommendations take into account all legal requirements for hygiene. In the German facilities, the central Head of Hygiene coordinates the hygiene specialists and establishes overarching standards, together with the Chief Medical Officer. One of the most important hygiene measures is hand disinfection.
Fresenius Vamed follows the guidelines of the WHO in this regard. Hygiene specialists, doctors, and nurses with special hygiene responsibilities implement hospital hygiene measures. In Austria, the Federal Hospitals Act forms the basis for the management of hygiene plans, hygiene inspections, the use of hygiene specialists, and doctors with special hygiene responsibilities. In the course of the COVID-19 pandemic, hygiene inspections in the facilities were intensified. Hand hygiene and the correct wearing of protective equipment were continuously addressed.
There have been outbreaks of COVID-19 in the facilities of VAMED Health Germany. According to the RKI, an outbreak or suspected outbreak exists as soon as a nosocomial case is detected. The local crisis team was immediately convened with the assistance of the consultant hospital hygienist. The central crisis team of Fresenius Vamed and the responsible public health department were also informed immediately. The measures to be taken were based on the extent of the outbreak and the other accompanying circumstances and always included at least extensive screening for SARS-CoV-2 in patients and staff. Other necessary measures, such as stopping admissions or transfers, restricting therapy, etc., were agreed in close consultation with those responsible on-site. If necessary, these measures were implemented before they were required by the health authorities.
Patient information
Fresenius Vamed provides information to its patients in different ways – for example, in the patient information folder or in the treatment contract, and via information brochures, privacy statements, the house rules, and the mission statement. Welcome lectures and training sessions are also offered. The website is available as a source of information before arrival. The goal of therapy is usually discussed and evaluated with patients during admission and discharge discussions.
Since Fresenius Vamed is also active as an accredited inspection body (ISO 17020) and as a manufacturer of medical gas supply systems (RL93 / 42 EEC), the business segment is subject to both a labeling obligation and an information obligation in accordance with RL93 / 42 EEC and MPG and / or ISO 13485. The accreditation authority uses external audits, for example, to check whether appropriate provisions exist and whether regulatory or normative requirements are complied with.
Our ambitions
Fresenius Vamed defines its quality goals annually with the aid of additional key performance indicators. The findings from complaint, case, and risk management are also incorporated.
The goals are reviewed regularly.
Progress and measures 2021
In the reporting year, the management approach and the governance structure of Fresenius Vamed remained as reported in 2020. Progress was focused on the safeguarding and application of hygiene and safety protocols and on adapting those to regulatory provisions.
Evaluation
Fresenius Vamed assesses the health and safety impacts of all significant product, treatment, and service categories for improvement potential.
Personalized and individually tailored rehabilitation goals
Fresenius Vamed uses modern, resource-oriented approaches, such as the ICF concept (International Classification of Functioning, Disability and Health) or the computer-based evaluation system CHES (Computer-Based Health Evaluation System). This enables patients to achieve the best possible, evidence-based functional improvement to increase activity and participation in all areas of life, even after severe illness.
In addition, the findings on treatment quality are published, for example by Fresenius Vamed Germany on the website Qualitaetskliniken.de. This allows patients to find out about key quality parameters of the various clinics before they are admitted.
Measurement of patient satisfaction and grievance processes
Fresenius Vamed measures patient satisfaction in its health care facilities in a continuous and structured process. The evaluation is conducted on a weekly and a monthly basis.
The company collects data, evaluates it internally, and implements appropriate measures, if necessary. Patient surveys are conducted while the patient is in the clinic, as well as after their rehabilitation; in some clinics both approaches are established. In this way, the clinics receive comprehensive feedback with regard to patient satisfaction.
Fresenius Vamed uses reporting systems for critical events and near-misses in its health care facilities, i. e., the electronic CIRS (Critical Incident Reporting System). Critical incidents can be reported anonymously there. The reports are processed by a dedicated committee. In addition, Fresenius Vamed uses systems for suggestions for improvement, material vigilance (material safety), and pharmacovigilance (drug safety). Thanks to these systems, a timely and appropriate response to potential sources of danger or complaints can be made, aligned with our internal quality standards.
Audits and recertification
To ensure adherence to quality standards, Fresenius Vamed also performs regular internal audits as well as external recertifications. This is done in the certified health care companies as well as in the other facilities of Fresenius Vamed. Quality management audits are carried out there once a year in accordance with the ISO regulations. Internal audits are carried out systematically and cover all business segments, and at a minimum, those topics that are required by the certified standards – i. e., all quality management processes. Besides ISO certifications, audits are conducted by the external regulatory bodies, listed in the chapter Well-being of the patient, section Certifications and commitment.

