Fresenius Kabi is a global health care company that specializes in lifesaving medicines and technologies. Our daily work has a major impact on patients’ quality of life. Our goal is to meet the needs of patients and health care professionals while driving forward the topic of sustainability in health care.

Major events
Vision 2026 defined and launched.
Vision 2026 is the framework to transform Fresenius Kabi for the next decade and defines a sustainable growth path. Fresenius Kabi will focus on three growth vectors in the future: broadening our biopharmaceutical offering; further introducing clinical nutrition products; expanding in MedTech. In the volume-driven IV business, we will continue to build our resilience.
Adjustment of organizational structure.
As part of Vision 2026, we want to improve our global competitiveness and the effectiveness of our organization. A first step in this process is the introduction of a business-oriented instead of a regional organization. In the new organizational structure, more responsibility will be assigned to the business segments and regions to support Fresenius Kabi’s growth targets, and the number of interfaces in the company will be reduced.
Important milestone towards approval of another biosimilar.
Positive news for our goal of improving treatment options for patients. MSB11456, a tocilizumab biosimilar candidate, successfully met primary and secondary endpoints in two consecutive clinical trials. New products serve a key role in the implementation of our strategic agenda for sustainable growth.
Collaboration to improve supply of key medicines to U.S. patients.
Together with the non-profit Phlow Corporation, we will improve access to affordable intravenous medicines for U.S. pediatric hospital patients. This is in line with our commitment to provide ever more people with state-of-the-art therapies and medicines.
Comprehensive product portfolio
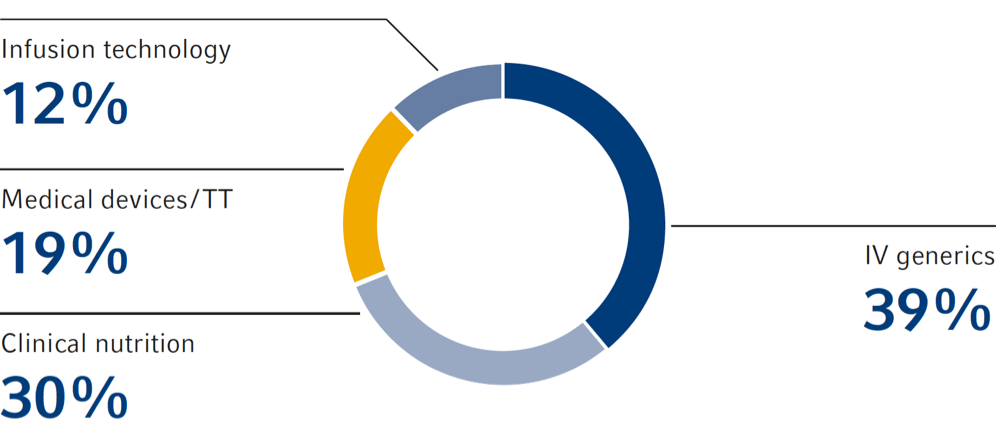
IV drugs
Intravenously administered generic drugs (IV) across a wide array of therapeutic categories: oncology, anesthetics, analgesics, anti-infectives, and critical care. This type of administration is used in cases of emergency, since the drug reaches the entire human body directly through the bloodstream and can be effective within a few seconds. IV drugs are also administered in intensive care and during surgery.
Clinical nutrition
Parenteral nutrition (administered intravenously) and enteral nutritionEnteral nutritionApplication of liquid nutrition as a tube or sip feed via the gastrointestinal tract. (administered as sip or tube feed via the gastrointestinal tract). Both serve to help patients who cannot eat any, or sufficient, normal food.
Infusion therapy
Infusion solutions and blood volume substitutes.
Biosimilars
A biosimilar is a biological medicine highly similar to another already-approved biological medicine (the “reference medicine”). Fresenius Kabi’s focus is on autoimmune diseases and oncology.
Medical devices
Devices and disposables used to administer IV generic drugs, infusion therapies, and clinical nutrition products.
Transfusion technology
Products for collection of blood components and extracorporeal therapies.
Our success factors
- Improve the quality of life of our patients
- Affordable high-quality products
- Supply reliability
- Highly qualified employees
- Strong global footprint
- Leading market positions
- Innovation in products, processes, and systems
Our growth drivers
- Organic growth through geographic product rollouts and product launches
- Development of qualified and talented employees
- Strong product pipeline
- Development and rollout of biosimilars
- Increasing presence in emerging markets
- Selective small and mid-sized acquisitions
Market dynamics
Continuing growth of generics in 2022 expected
high single-digit
% range
for generic IV drugs
(in sales/€)
Continuing growth of biopharmaceuticals in 2022 expected
4 – 6%
(in volume)
Rising cost consciousness in health care spending – significant savings from generics
~ US$ 313 bn
savings p.a.
in the United States
Expected market growth of biosimilarsBiosimilarsA biosimilar is a drug that is “similar” to another biologic drug already approved. 2021 to 2028
+27%
average growth p.a.
in the United States
Global addressable market 2021
~ € 114 bn
Growing health care spending in emerging markets
+6.3%
growth p.a.
over the next decade
Increase of population older than 60 years to
1.4 bn
in 2030
(2020: 1 bn)
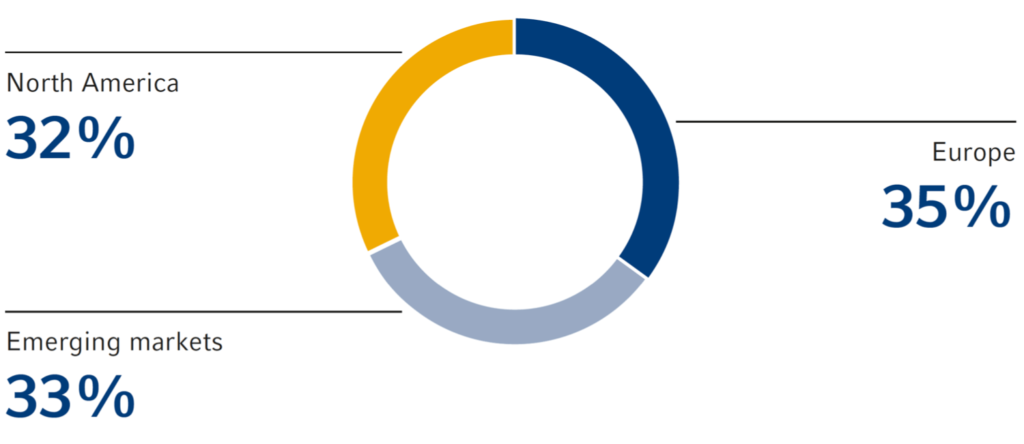
Sales by region
Sales by product segment
Sales and earnings development
North America
Download(XLS, 35 KB)| in € millions | 2021 | 2020 | Change | Change in constant currency |
|---|---|---|---|---|
| Sales | 2,258 | 2,376 | -5% | -2% |
| EBIT | 637 | 785 | -19% | -16% |
| Employees | 9,815 | 8,809 | 11% |
Emerging Markets
Download(XLS, 35 KB)| in € millions | 2021 | 2020 | Change | Change in constant currency |
|---|---|---|---|---|
| Sales | 2,391 | 2,142 | 12% | 12% |
| EBIT | 646 | 471 | 37% | 38% |
| Employees | 16,276 | 16,586 | -2% |
Europe
Download(XLS, 35 KB)| in € millions | 2021 | 2020 | Change | Change in constant currency |
|---|---|---|---|---|
| Sales | 2,544 | 2,458 | 3% | 3% |
| EBIT | 374 | 355 | 5% | 5% |
| Employees | 15,306 | 15,124 | 1% |
Sources:
Continuing growth of generics and biopharmaceuticals in 2022 expected
Source: own research Fresenius Kabi
Global addressable market 2021 ~ € 114 bn
Source: own research Fresenius Kabi
Increase of the population older than 60 years
Source: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Highlights.pdf
Rising cost consciousness in health care spending – significant savings from generics
Source: Association for Accessible Medicines (AAM): 2019 Generic Drug and BiosimilarsBiosimilarsA biosimilar is a drug that is “similar” to another biologic drug already approved. Access and Savings in the U.S.; IMS Health 2015, The Role of Generic Medicines in Sustaining Healthcare Systems – A European Perspective
Expected market growth of biosimilars 2021 to 2028
Source: https://www.fortunebusinessinsights.com/industry-reports/u-s-biosimilars-market-100990
Growing health care spending in emerging markets
Source: UBS, Longer Term Investments: EM healthcare (2018)

