Product and process development and the improvement of therapies are at the core of our growth strategy. Fresenius focuses its R & D efforts on its core competencies in the fol- lowing areas:
- Dialysis
- Generic IV drugs
- Biosimilars
- Infusion and nutrition therapies
- Medical devices
Apart from new products, we are concentrating on developing optimized or completely new therapies, treatment methods, and services.
Research services provided by third parties are mainly used by Fresenius Kabi, especially in the field of biosimilars. Detailed figures are provided in the Notes.
As of December 31, 2021, there were 3,656 employees in research and development (2020: 3,565). Of that number, 1,236 were employed at Fresenius Medical Care (2020: 1,262) and 2,366 at Fresenius Kabi (2020: 2,288).
Our main research sites are in Europe, the United States, and India. Product-related development activities are also carried out in China.
Research and development expenses1 were €818 million (2020: €748 million), approximately 7.5% of our product sales (2020: 7.2%).
1 Before expenses related to the Fresenius cost and efficiency program and revaluations of contingent biosimilarsBiosimilarsA biosimilar is a drug that is “similar” to another biologic drug already approved. purchase price liabilities
Key Figures Research and Development
Download(XLS, 36 KB)| 2021 | 2020 | 2019 | 2018 | 2017 | |
|---|---|---|---|---|---|
| R & D expenses, € in millions1 | 818 | 748 | 677 | 649 | 538 |
| as % of product sales1, 2 | 7.5% | 7.2% | 6.8% | 6.7% | 5.7% |
| R & D employees | 3,656 | 3,565 | 3,412 | 3,042 | 2,772 |
| 1 2021: Before expenses related to the Fresenius cost and efficiency program and revaluations of contingent biosimilars purchase price liabilities 2020, 2019, and 2018: Revaluations of biosimilars contingent purchase price liabilities |
|||||
| 2 2021, 2019, and 2018 excluding impairment losses from capitalized in-process R & D activities |
|||||
r&d expenses by segment
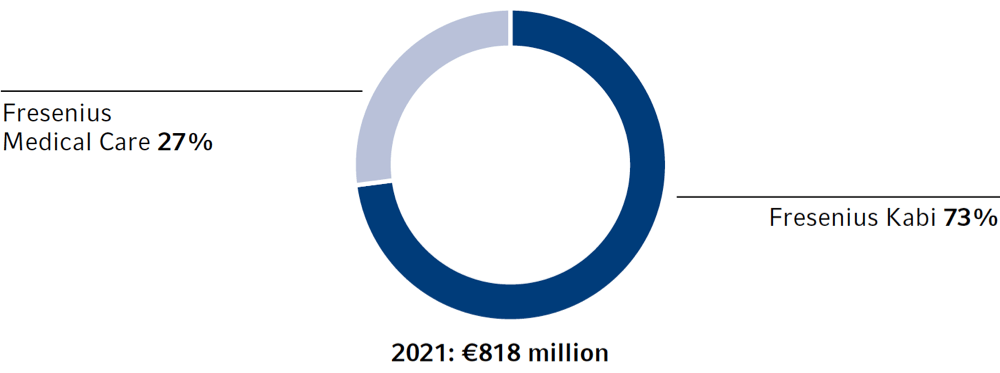
1 Before expenses related to the Fresenius cost and efficiency program and revaluations of contingent biosimilars purchase price liabilities
Fresenius Medical Care
Our aim is to continuously improve our patients’ quality of life and their treatment. Our intention is therefore to drive the development of new products through to market launch and to expand our extensive portfolio of innovation projects. These focus on technologies in our core business as well as related areas of strategic interest.
We intend to deliver innovative, competitive products and further strengthen our focus on developing countries.
In addition to research and development activities within our company, we collaborate with external partners with the aim of building a comprehensive innovation and technology network. These partners include numerous academic institutions, such as research institutes at prestigious universities in the United States. Another is the Renal Research Institute (RRI) in New York. This subsidiary of Fresenius Medical Care North America is a renowned institution in the field of clinical research into all aspects of chronic kidney failure. Together, we are working on fundamental issues relating to renal therapies. We are also increasingly collaborating with start-ups and early-stage companies with the objective of promoting innovation and enabling access to the latest technologies.
The COVID-19 pandemic had no major influence on our R & D activities. In 2021, we continued with our research and development work.
The new FX CorAL dialyzerDialyzerSpecial filter used in hemo dialysis for removing toxic substances, waste products of metabolic processes, and excess water from the blood. The dialyzer is sometimes referred to as the “artificial kidney”. was officially presented at the virtual ERA-EDTA Congress (European Renal Association-European DialysisDialysisForm of renal replacement therapy where a semipermeable membrane – in peritoneal dialysis the peritoneum of the patient, in hemo dialysis the membrane of the dialyzer – is used to clean a patient’s blood. and Transplant Association Congress) in June 2021. The FX CorAL was developed with a focus on clinical performance and hemo-compatibility, both important factors in patient-oriented dialysis. The dialyzer is based on the innovative Helixone hydro® membrane, which forms a hydrous film on the inner membrane surface. This reduces protein adsorption, resulting in a weakened immune response and high selective permeability of the membrane. The aim here is to reduce the side effects of dialysisDialysisForm of renal replacement therapy where a semipermeable membrane – in peritoneal dialysis the peritoneum of the patient, in hemo dialysis the membrane of the dialyzer – is used to clean a patient’s blood. treatment. The multiFiltratePRO is a highly innovative platform for continuous renal replacement therapy. It offers advanced features such as renal replacement therapy incorporating proven regional Ci-Ca® citrate anticoagulation and therapeutic plasma exchange. The multiFiltratePRO has received emergency approval in the United States and was launched in China and several South American countries in 2020, so it now has a broad base in the market. In 2022, we aim to grow further and implement optimization measures based on the significant increase in the number of devices on the market and increased sales; the development of the corresponding measures was driven forward in 2021 and is now almost complete.
Fresenius Kabi
Fresenius Kabi’s research and development activities concentrate on products for the therapy and care of critically and chronically ill patients. Our products are used where the patient is most at risk: in emergency medicine, intensive care, special care, and in those who need to be treated in hospital or as an outpatient for a longer period of time. In these patient groups, every single step is essential for the success of the therapy. Products make a crucial contribution to the success of the treatment and the interaction between medicine and technology is highly important.
We consider it our task to develop products that help to support medical advancements in acute and post-acute care and improve patients’ quality of life. At the same time, our products are intended to enable an increasing number of people worldwide to have access to high-quality, modern therapies.
Chronic diseases are on the increase worldwide; more and more people need access to high-quality therapies. In the care of critically ill patients, the requirements for successful treatment are becoming ever higher. The demand for effective therapies in conjunction with intelligent medical technology applications and devices will continue to rise in the future. We want to be the preferred point of contact for doctors and nursing staff in the care of critically and chronically ill patients. For this purpose, Fresenius Kabi Vision 2026 was developed during the reporting year and adopted in the fourth quarter of 2021. With Vision 2026, we have defined a clear direction for Fresenius Kabi with three growth paths: the broadening of our biopharmaceutical range, the further development and global introduction of our clinical nutrition products, and expansion in the area of MedTech. In the volume-driven IV business, we will continue to expand our resilience. Our future development work will be geared toward this.
Our development expertise includes all the related components, such as the drug raw material, the pharmaceutical formulation, the primary packaging, the medical device needed for application of drugs and infusions, and the production technology. In the area of biosimilars, we have specialized in the development of products in the areas of autoimmune diseases and oncology.
In the area of generic IV drugs, we are continuously working on the extension of our product portfolio. What matters most to us here is that we launch new generic drug formulations directly after the patents of the branded products expire. For example, in the reporting year, we launched the cancer drug pemetrexed 25 mg/ml concentrate for the preparation of an infusion solution in Europe on June 1, therefore bringing it onto the market two weeks before the patent expired, based on a patent agreement with the original manufacturer. We also introduced the foscarnet sodium injection in the United States; when it was launched in February 2021, our product was the only generic for the originator product Foscavir on the market. Foscarnet is mainly used to treat immunosuppressed patients. In addition, we are working on the continuous improvement of IV drugs already on the market. For example, we are developing IV drugs with new formulations and dosage forms, as well as improved primary packaging. In 2021, we had more than 100 active projects in the area of generics. We focus, among other things, on complex formulations such as active ingredients in liposomal¹ solutions and product improvements that bring added value to both medical staff and patients.
1 Liposomes are small vesicles consisting of a lipid bilayer and an aqueous core and are used to transport active substances in the body.
Thus, we develop ready-to-use products that are especially convenient and safe and help to prevent application errors in day-to-day medical care. These include ready-to-use solutions in our freeflex infusion bags, the cost-effective KabiPac infusion bottle and pre-filled syringes. Drugs with pre-filled syringes are simpler and safer to use than traditional applications. In the reporting year, for example, we introduced fentanyl in a pre-filled syringe in the United States.
In the area of biosimilars, we have a pipeline of molecules at various stages of development focused on autoimmune diseases and oncology. A biosimilar is a biological product that is very similar to another approved biological product called a “reference product”. The biosimilar product corresponds to the reference product in terms of efficacy and safety. Since the existence of biosimilars, more and more patients have been treated with biopharmaceutical drugs. For many, biopharmaceutical therapy means a completely new life. Not only are more people being treated with biopharmaceuticals, but waiting times for them have also been significantly reduced in recent years.
We apply the same high quality standards to our biosimilar products during development and preparation as are required for the reference product. With our biosimilars, we offer more patients worldwide access to affordable, high-quality medicines.
Our research and development center for biosimilars is based in Eysins, Switzerland, where new products for the treatment of autoimmune and oncological diseases are developed in state-of-the-art development and research laboratories.
Our first biosimilar is Idacio1, an adalimumab biosimilar that can be used in chronic inflammatory diseases such as rheumatoid arthritis, Crohn’s disease and psoriasis (skin disease).
Since its introduction in 2019, we have launched the product in numerous countries within Europe, Latin America, and Asia-Pacific, as well as in Israel and Canada. In the reporting year, we worked on further marketing authorizations.
The clinical development of MSB 114552, a biosimilar candidate of pegfilgrastim, has been successfully completed and marketing authorization applications are currently being reviewed by the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDAFDA (U.S. Food & Drug Administration)Official authority for food observation and drug registration in the United States.) to obtain approval for market launch. MSB 11455 is a molecule that stimulates the growth of certain white blood cells. These blood cells are essential for fighting infections, which are a common side effect in cancer patients receiving chemotherapy. Approval for U.S. market launch requires the FDA to conduct inspections at the European production sites for MSB 11455. In the reporting year, the FDA informed Fresenius Kabi that it was postponing the completion of its review process, as COVID-19-related restrictions are delaying FDA inspections across the world. As a result, the FDA has also postponed its authorization decision until the inspections can be carried out and completed.
MSB 114563 is a biosimilar candidate of tocilizumab used in various indications such as rheumatoid arthritis. The clinical trial for MSB 11456, conducted with healthy volunteers, reached its primary endpoint in 2019 and demonstrated bioequivalenceBioequivalenceThe active pharmaceutical ingredient of the generic product is the same as that of the reference product. Both are therefore interchangeable with each other. for all pharmacokinetic parameters. In the reporting year, we were able to successfully complete our next development step for the market launch. Our biosimilar candidate MSB11456 showed positive results in two consecutive phase I studies and successfully achieved primary and secondary endpoints. In both studies, subcutaneous and intravenous formulations were used to cover the two different dosage forms. The bioequivalence, safety, and immunogenicityImmunogenicityThe ability of an antigen to cause an immune response (immunization, sensitization). of the biosimilar candidate of tocilizumab compared to its reference product were examined.
The first phase I study, a randomized, double-blind, parallel-group study to determine the pharmacokineticsPharmacokineticsThe effect of the body on the drug. , pharmacodynamicsPharmacodynamicsThe effect of the drug on the body., safety, tolerability, and immunogenicity of MSB11456 met all primary and secondary endpoints following a single subcutaneous injectionSubcutaneous injectionAn injection of vaccines or drugs into the subcutaneous fat tissue. in healthy volunteers. The pharmacokinetic equivalence of MSB11456 with the U.S. reference product4 and the EU reference drug5 was demonstrated successfully. The clinical study confirmed the similarity of MSB11456 with the U.S. reference product4 and the EU reference drug5 at the pharmacodynamic level. No significant differences in safety and immunogenicity were found between the three treatment groups (MSB11456, U.S. reference product4, and EU reference drug5).
1 Idacio is a biosimilar of Humira® and has not yet been approved by the relevant health authorities. Humira® (adalimumab) is a registered trademark of AbbVie Biotechnology Ltd.
2 MSB 11455 is a biosimilar candidate of Neulasta® and has not yet been approved by the relevant health authorities. Neulasta® (pegfilgrastim) is a registered trademark of Amgen Inc.
3 MSB 11456 is a tocilizumab biosimilar candidate of Actemra®/RoActemra® and has not yet been approved by the relevant health authorities. Actemra®/RoActemra® (tocilizumab) are registered trademarks of Chugai Seiyaku Kabushiki Kaisha.
4 U.S. reference product Actemra®.
5 EU reference product RoActemra®.
In the second phase I study, all primary and secondary endpoints were also achieved and the pharmacokinetic equivalence of MSB11456 and the U.S. reference product1 was successfully demonstrated. This clinical study was also a randomized, double-blind, parallel-group study to investigate the pharmacokinetics, safety, immunogenicity, and tolerability of MSB11456 compared to its U.S. reference product1 following a single intravenous infusion in healthy volunteers. No significant differences in safety and immunogenicity were found between the two treatment groups (MSB11456 and U.S. reference product1).
1 U.S. reference product Actemra®.
In 2020, we also launched a global phase III study to compare the efficacy, safety, tolerability, and immunogenicity of our biosimilar candidate of tocilizumab, MSB 11456, with the EU reference product in patients with moderately to severely active rheumatoid arthritis. The inclusion of patients from several European countries in this study was completed in 2021.
Clinical nutrition provides care for patients who cannot nourish themselves normally or sufficiently. This includes, for example, patients in intensive care and those who are seriously or chronically ill. Early and correct intervention can help prevent malnutrition and its consequences.
Malnutrition is a common indication in hospitalized patients: studies carried out in hospitals in Europe show that one in four patients in the hospital suffers from malnutrition or is at risk of malnutrition. The clinical significance of malnutrition results from a less favorable prognosis in terms of morbidity and mortality. Further consequences can be a longer stay in hospital and higher treatment costs.
In the parenteral nutritionParenteral nutritionApplication of nutrients directly into the bloodstream of the patient (intravenously). This is necessary if the condition of a patient does not allow them to absorb and metabolize essential nutrients orally or as sip and tube feed in a sufficient quantity. product segment, we focus our research and development on products that make a significant contribution to improving clinical treatment and the nutritional condition of patients and on innovative containers such as our multi-chamber bags that are safer and more convenient in everyday use.
In the reporting year, we completed our EuroPN study and presented the first results to health care professionals at international scientific congresses. The study examined clinical nutritional practice in more than 1,000 critically ill patients in Europe. EuroPN is currently the largest longitudinal study conducted that examines the effects of nutritional targets on the success of treatment of critically ill patients who have been treated in an intensive care unit for more than five days. The results are due to be published in 2022.
In addition to our own development, Fresenius Kabi also supports external development projects that contribute to improving the nutritional care of critically ill patients and surgery patients. In 2019, we initiated the funding program “Jumpstart”. With this program, we support the research work of young scientists and doctors in clinics in the area of parenteral nutrition. An independent jury, consisting of internationally renowned scientists in the area of clinical nutrition, is responsible for selecting the fellows and awarding the research prize.
In the reporting year, we continued the funding program and awarded another research prize at the ESPEN international congress on nutrition in September 2021.
In 2021, we also continued development work on parenteral products. We are concentrating on formulations that are tailored to the needs of individual patient groups. In addition to our global development projects, we are also working on parenteral nutrition products for specific markets such as the United States, China, and Europe.
One focus area is on the use of fish oil in parenteral nutrition. Parenteral nutrition containing fish oil has numerous beneficial effects on important biological functions, including the modulation of the immune and inflammatory response. The use of fish oil in parenteral nutrition products helps to improve clinical outcomes such as infection rates, sepsis rates, and the duration of stays in the ICU and in hospital in general.
In the area of enteral nutritionEnteral nutritionApplication of liquid nutrition as a tube or sip feed via the gastrointestinal tract., we are focusing our research and development activities on product concepts that support therapeutic compliance and thus the success of therapy.
In particular, the flavor of enteral products is known to be a critical parameter in ensuring the acceptance of the products and compliance with the nutritional therapy. For years, we have been working continuously to develop products with a wide variety of flavors to offer the users variations and thus provide them with the best possible support to complete the necessary nutritional therapy. Another focus of our work is on the development of products with an increased calorie and protein concentration. This way, we make it easier for the user to take in the necessary amount of nutrients in small volumes. In addition to global product developments, we are continuing to work on product developments for specific market requirements. The main focus here is on products for markets with high growth potential, such as for Taiwan and Korea in Asia-Pacific and Brazil in Latin America.
In the area of infusion solutions, we are continuously working on new primary containers and containers that have already been introduced with the aim of increasing efficiency and safety in everyday hospital life and facilitating their use. In the reporting year, we began introducing our optimized freeflex+ infusion bag with needle-free injection port. The aim of this product is to help reduce the risk of potential injuries in daily hospital life. Furthermore, we are continuously working on expanding our product range, which we want to introduce globally. We are also developing products that will allow us to further tap into the U.S. market by means of local production.
In the area of medical devices, we focus on developing new products as well as on further developing our existing portfolio. This industry in particular is characterized by technological innovations. Digitalization is a more crucial factor here than in any of our other product segments. Devices not only have to be continuously developed in terms of their application, but they also have to increasingly be embedded in the IT system landscape of hospitals, blood donation centers, and plasma centers.
In the future, we want to benefit from this trend and are already focusing on the continuous development of our software solutions to increase the efficiency and clinical outcome for our customers.
In the reporting year, we continued to develop the Vigilant Software Suite, a software solution for our Agilia family of infusion pumps in hospitals. As a comprehensive, server-based therapy information system, it combines the central monitoring of our pumps (Vigilant Sentinel), the central administration of the drug databases required for the patient (Vigilant Mastermed), comprehensive quality reporting (Vigilant Insight), and a standardized IT interface application (Vigilant Bridge). In addition to further development, we are also pushing ahead with the international market launch through further language versions, regulatory work, and support for the installation of the devices in hospitals.
We also continued the development work on a new infusion management system in the reporting year. This system features a modern operating system and will enable new therapy and treatment procedures in the intensive care unit and operating room.
With regard to disposable medical products, the focus was on developing components that increase the safety and simplicity of use of the products and enable therapy with new drugs.
In research and development in the area of transfusion technology, we are working intensively on products for use in cell therapy. Our focus is on product developments for the automated washing and concentration of cell concentrates. These products are used in CAR T-cell¹ and similar cell therapies. In 2021, we started preparations for the market launch of the CUE1 cell processing system. This device has been specifically developed for smaller filling quantities and end-use applications in the area of cell therapy and will complement our LOVO1 cell processing system, which is already available on the market. We will start the launch of CUECUECue is an automated cell processing system capable of washing, concentrating, and preparing white blood cell suspensions for cryopreservation (freezing in liquid nitrogen) and/or dispensing into final containers. in 2022.
1 For more information, see the Glossary.
In the area of extracorporeal photopheresis (ECP), we continue to focus on the introduction of the Amicus Blue system and the associated Phelix light box in Europe, as well as on the further development of an ECP application method, which only requires one vascular access. In this therapy method, certain blood cells outside the body are treated with ultraviolet light (phototherapy). This method is used to treat various immunological diseases, including to kill malignant immune cells (lymphocytes) outside the body.
Another focus area is the continuous further development of our devices and the corresponding data management software. This includes the transfusion technology devices for plasma (Aurora Xi/optimized Nomogram Software), blood processing (Compoguard), and platelets (AmiCORE/COM tec advanced).

