New product and process development and the improvement of therapies are at the core of our strategy. Fresenius focuses its R & D efforts on its core competencies in the following areas:
- Dialysis
- Generic IV drugs
- Biopharmaceuticals
- Infusion and nutrition therapies
- Medical devices
Apart from new products, we are concentrating on developing optimized or completely new therapies, treatment methods, and services.
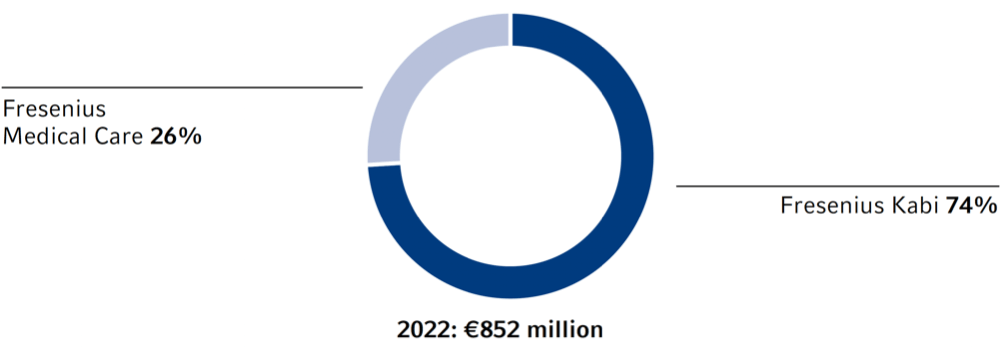
Research services provided by third parties are mainly used by Fresenius Kabi, especially in the field of biopharmaceuticals.
R & D expenses by segment1
1 Before expenses related to the Fresenius cost and efficiency program and revaluations of contingent biosimilarsBiosimilarsA biosimilar is a drug that is “similar” to another biologic drug already approved. purchase price liabilities
As of December 31, 2022, there were 3,799 employees in research and development (2021: 3,656). Of that number, 1,235 were employed at Fresenius Medical Care (2021: 1,236) and 2,525 at Fresenius Kabi (2021: 2,366).
Our main research sites are in Europe, the United States, and India. Product-related development activities are also carried out in China.
Research and development expenses1 were €852 million (2021: €818 million), approximately 7.2% of our product revenue (2021: 7.5%).
1 2022, 2021: Before expenses related to the Fresenius cost and efficiency program; 2021 before revaluations of contingent biosimilars purchase price liabilities
Key Figures Research and Development
Download(XLS, 36 KB)| 2022 | 2021 | 2020 | 2019 | 2018 | |
|---|---|---|---|---|---|
| R & D expenses, € in millions1 | 852 | 818 | 748 | 677 | 649 |
| as % of product revenue1, 2 | 7.2% | 7.5% | 7.2% | 6.8% | 6.7% |
| R & D employees | 3,799 | 3,656 | 3,565 | 3,412 | 3,042 |
| 1 2022, 2021: before expenses related to the Fresenius cost and efficiency program 2021, 2020, 2019, and 2018: before revaluations of biosimilars contingent purchase price liabilities |
|||||
| 2 2022, 2021, 2019, and 2018 excluding impairment losses from capitalized in-process R & D activities | |||||
Fresenius Medical Care
At Fresenius Medical Care, we are driving the development of new products that continuously improve the quality of life and treatment outcomes of our patients. We have an extensive portfolio of innovation projects.
We focus on technologies in both our core business and related areas that are of strategic interest to us.
In the future, we want to provide innovative, competitive products even more efficiently. As part of our organizational realignment, we have therefore begun to consolidate our previously decentralized product business, including research and development, in the Care Enablement segment as of January 1, 2023. The product business will be organized according to the three treatment modalities we offer: dialysisDialysisForm of renal replacement therapy where a semipermeable membrane – in peritoneal dialysis the peritoneum of the patient, in hemo dialysis the membrane of the dialyzer – is used to clean a patient’s blood. centers, home dialysis, and critical care.
Alongside our research and development activities, we collaborate with external partners with the aim of expanding our comprehensive innovation and technology network. These partners include numerous academic institutions, such as research institutes at prestigious universities in the United States. Another partner is the Renal Research Institute in New York. This subsidiary of Fresenius Medical Care North America is a renowned institution in the field of clinical research into all aspects of chronic kidney failure. Together, we are working on fundamental issues relating to renal therapies. In addition, Fresenius Medical Care Ventures collaborates with start-ups and early-stage companies with the objective of promoting an open culture of innovation and enabling access to the latest technologies.
In-center dialysis
The FDAFDA (U.S. Food & Drug Administration)Official authority for food observation and drug registration in the United States. has approved the 2008-series hemodialysis machines with silicon tubing, which includes platinum catalysts. The platinum catalyst tubing eliminates detectable non-dioxin-like (NDL) polychlorinated biphenyl acids (PCBAs) in machines in this series and thus addresses the concerns raised by the FDA in May 2022.
Home dialysis
For many people with chronic kidney failure, peritoneal dialysis is the gentlest and thus preferred treatment option during the first years of renal replacement therapy. Our goal for this form of treatment is to make therapy systems more accessible, intelligent, and networked. One example of this is the Kinexus digital therapy platform, which will support all APD (automated peritoneal dialysis) cyclers in our portfolio in the future and provide physicians and nurses with continuous online access to treatment data and remote programming of individual prescriptions. This will further improve treatment outcomes and increase the productivity of the caregivers deployed. The Kinexus platform is already available in conjunction with the Liberty® Select Cycler, a peritoneal dialysis machine used in the U.S. market that received FDA approval for additional programming through remote therapy in November 2022. This digital innovation is expected to reduce hospitalizations, technical failures and treatment discontinuations. It also extends the average length of stay on peritoneal dialysis, which is typically beneficial for patients. In addition, Kinexus will serve as an enabling technology for future innovations.
SILENCIA is a new APD cycler that uses a very simple, ultra-quiet, and highly reliable gravity-based fluid control mechanism that enables high-quality automated peritoneal dialysis to be performed at very low cost. Positive results in terms of stability and functionality of the system have already been achieved in treatments in South America. A rollout in Asia, the Middle East, and North Africa is planned.
In fiscal year 2022, we launched the “China CAPD” app (CAPD = continuous ambulatory peritoneal dialysis) on the Chinese market. The app is designed to help peritoneal dialysis patients enter therapy data and vital signs, order consumables and track order progress and delivery themselves. The China CAPD app enables medical professionals to gain an improved overview of therapy outcomes, document home visits and provide targeted training content for their patients.
Critical care
Continuous kidney replacement therapy (CKRT), in which the blood is purified by means of special solutions and filters, is a proven and effective treatment option for patients with acute kidney damage. The natural functions of the kidney are imitated and continuous monitoring of body fluid balance is enabled.
Another leading CKRT platform, the NxStage System One, is available in the United States. Its “speed swap” function, launched in 2022, enables filter replacement during therapy without changing the treatment set. This new option makes the therapy system more attractive for day-to-day use by the clinic staff.
Digitalization in healthcare
Digitalization of processes in healthcare is mainly focused on connecting patients, doctors, and nursing staff and improving nursing documentation at the point of care. The aims are achieve better treatment results and significant reductions in treatment costs for our patients as well as an improvement in our own cost base.
Connected patient care will make it possible to coordinate treatments individually and detect warning signs and causes of kidney disease at an early stage. To this end, using the world’s largest database for clinical data in the field of advanced kidney disease, we are developing modules based on artificial intelligence and machine learning in order to assist doctors and nursing staff with their duties.
Research in the field of regenerative medicine
We have further expanded our collaboration with the U.S. pharmaceutical company Humacyte, Inc. (Humacyte), a developer and manufacturer of universally implantable biotechnologically produced human tissue.
The Humacyte Human Acellular Vessel (HAV) is a regenerative vascular system used for various vascular applications, including vascular trauma repair, arteriovenous access for hemodialysis, and peripheral arterial disease. Our investment in Humacyte is currently centered on the most advanced clinical program, with market launch having occurred in under two years.
Fresenius Medical Care Ventures
Fresenius Medical Care Ventures makes targeted investments in start-ups and early-stage companies in the fields of diagnostics, therapies, medical equipment, digital solutions, xenotransplantation, and monitoring technologies. The aim is to improve treatment outcomes for patients suffering from chronic diseases or in need of acute care.
Fresenius Kabi
Fresenius Kabi’s research and development activities concentrate on products for the therapy and care of critically and chronically ill patients. Our products are used where the patient is most at risk: in emergency medicine, intensive care, special care, and for those who need to be treated in hospital or as an outpatient for a longer period of time. In these patient groups, every single step is essential for the success of the therapy. Products from Fresenius Kabi make a crucial contribution to the success of the treatment, and the interaction between medicine and technology is highly important.
We consider it our task to develop products that help to support medical advancements in acute and post-acute care and improve patients’ quality of life. At the same time, our products are intended to enable an increasing number of people worldwide to have access to high-quality and modern therapies.
Chronic diseases are on the increase worldwide. In the care of critically ill patients, the requirements for successful treatment are becoming ever higher. The demand for effective therapies in conjunction with intelligent medical technology applications and devices will continue to rise in the future. We want to be the preferred point of contact for doctors and nursing staff in the care of critically and chronically ill patients. With Fresenius Kabi’s Vision 2026 we have defined a clear direction for Fresenius Kabi with three growth paths: broadening of our biopharmaceutical range, further development and global introduction of our clinical nutrition products, and expansion in the area of MedTech. In the volume-driven IV business, we will continue to expand our resilience. Our future development work will be geared toward this.
Our development competence includes all related components, such as the drug raw material, the pharmaceutical formulation, the primary packaging, the medical device needed for application of drugs and infusions, and the production technology. In the area of biopharmaceuticals, we have specialized in the development of products in the areas of autoimmune diseases and oncology.
In the biopharmaceutical area we are committed to bringing more therapeutic solutions to more patients. In both therapeutic areas, we continue to expand our biosimilar portfolio and have multiple candidates in various development phases. A biosimilar is a biological product that is similar to another approved biological product called a “reference product”. The biosimilar product demonstrates a similar analytical profile, pharmacokineticsPharmacokineticsThe effect of the body on the drug., efficacy, safety, and immunogenicityImmunogenicityThe ability of an antigen to cause an immune response (immunization, sensitization). to the reference product. The adoption and uptake of biosimilars has been growing worldwide and more and more patients have been treated with high-quality biologic drugs. For many, biologic therapy means a completely new life, and access to biologics has been improved in recent years.
With our growing biosimilars portfolio, we offer more patients worldwide access to high-quality, safe, effective, and affordable medicines. We apply the same high-quality standards to our biosimilar products during research, development, and preparation as are required for the reference product.
Our research and development center for biosimilars is based in Eysins, Switzerland, where new biosimilars for the treatment of autoimmune and oncological diseases are developed in state-of-the-art development and research laboratories. Furthermore, Fresenius Kabi acquired a majority stake in mAbxience, a leading international biopharmaceutical company, in 2022.
Our first biosimilar is Idacio1, an adalimumab biosimilar that can be used in chronic inflammatory diseases such as rheumatoid arthritis, Crohn’s disease, and psoriasis (skin disease). Since its introduction in 2019, we have launched the product in over 37 countries within Europe, the Middle East, North America, Latin America, and Asia-Pacific. In the reporting year, we worked on further market approvals. In December 2022, Fresenius Kabi received U.S. Food and Drug Administration (FDA) approval for Idacio.
Our second biosimilar, Stimufend®2, is a pegfilgrastim biosimilar, a drug used to treat patients who experience neutropenia following chemotherapy. It stimulates the growth of certain white blood cells, which are essential for fighting infections. The European Commission (EC) granted marketing authorization for Stimufend® for all approved indications of the reference medicine in the first quarter of 2022. In the reporting year, we have launched Stimufend® as planned in France and the FDA also granted approval for our pegfilgrastim biosimilar Stimufend® in September 2022.
MSB114563 is a biosimilar candidate of tocilizumab used in various indications such as rheumatoid arthritis. In the reporting year, Fresenius Kabi also announced that the FDA had accepted for review its 351(k) Biologics License Application (BLA) for MSB11456, the company’s biosimilar candidate for Actemra® (tocilizumab). The BLA application includes presentations for both subcutaneous (prefilled syringe and autoinjector) and intravenous administrations designed to provide a comprehensive offering for patients using tocilizumab.
The tocilizumab biosimilar candidate is the company’s third BLA submission to the FDA, after submissions for its pegfilgrastim and adalimumab biosimilar candidates.
In August 2022, Fresenius Kabi additionally announced that the Marketing Authorization Application (MAA) for its tocilizumab biosimilar candidate (MSB11456) of RoActemra® has been accepted for review by the European Medicines Agency (EMA). The tocilizumab biosimilar candidate represents the third biosimilar candidate from Fresenius Kabi submitted in the European Union.
In addition to Fresenius Kabi’s biosimilars development activities in Switzerland, mAbxience develops biosimilar products in Spain and Argentina. Bevacizumab and rituximab, two of mAbxience’s biosimilars used to treat various types of cancer, have been commercially available to patients in more than 40 countries in Latin America, Asia-Pacific, and the Middle East for many years. In addition, mAbxience received EMA approval for its bevacizumab biosimilar in 2021, making it available in Europe.
In the United States, the FDA granted marketing approval for mAbxience’s bevacizumab biosimilar, making it available to patients in the United States as of October 2022. Further, mAbxience also received marketing approval for the bevacizumab biosimilar in five additional countries between August and December 2022.
1 Idacio is a biosimilar of Humira® and has not yet been approved by the relevant health authorities. Humira® (adalimumab) is a registered trademark of AbbVie Biotechnology Ltd.
2 Stimufend® (pegfilgrastim) is a registered trademark of Fresenius Kabi Deutschland GmbH in selected countries; it is a pegfilgrastim biosimilar of Neulasta® (pegfilgrastim) a registered trademark of Amgen Inc.
3 MSB11456 is a tocilizumab biosimilar candidate of Actemra®/ RoActemra® and has not yet been approved by the relevant health authorities. Actemra®/ RoActemra® (tocilizumab) are registered trademarks of Chugai Seiyaku Kabushiki Kaisha Corp., a member of the Roche Group.
Clinical nutrition provides care for patients who cannot nourish themselves normally or sufficiently. This includes, for example, patients in intensive care units (ICU) and those who are seriously or chronically ill. Early and adequate intervention can help prevent malnutrition and its consequences.
Malnutrition is a common problem in hospitalized patients: studies carried out in Europe show that one in four patients in the hospital suffers from malnutrition or is at risk of malnutrition. The clinical significance of malnutrition results from a less favorable prognosis in terms of morbidity and mortality. Further consequences can be a longer stay in the hospital and higher associated treatment costs.
In the parenteral nutrition product segment, we focus our research and development on products that help improve clinical treatment and the nutritional condition of patients, and on innovative containers such as our multi-chamber bags that are safe and convenient in everyday use.
In 2022, we also continued development work on parenteral products. We are concentrating on formulations that are tailored to the needs of individual patient groups. In addition to our global development projects, we are also working on parenteral nutrition products for specific markets such as the United States, China, and Europe.
One focus area is the use of fish oil in parenteral nutritionParenteral nutritionApplication of nutrients directly into the bloodstream of the patient (intravenously). This is necessary if the condition of a patient does not allow them to absorb and metabolize essential nutrients orally or as sip and tube feed in a sufficient quantity.. Parenteral nutrition containing fish oil has numerous beneficial effects on important biological functions, including the modulation of the immune and inflammatory response. The use of fish oil in parenteral nutrition products may help to improve clinical outcomes and the duration of ICU and hospital stays.
In the area of enteral nutritionEnteral nutritionApplication of liquid nutrition as a tube or sip feed via the gastrointestinal tract., we are focusing our research and development activities on product concepts that support therapeutic compliance and thus the success of therapy. In particular, the flavor of enteral products is known to be a critical parameter in ensuring the acceptance of the products and compliance with the nutritional therapy. For years, we have been working continuously to develop products with a wide variety of flavors to offer the users variations and thus provide them with the best possible support to complete the necessary nutritional therapy. In this regard, we are expanding our offer of plant-based products as a response to customers’ demands and preferences. Another focus of our work is on the development of products with an increased calorie and protein concentration. This way, we make it easier for the user to take in the necessary amount of nutrients in small volumes. In addition to global product developments, we are continuing to work on product developments for specific market requirements.
In the area of medical devices, we focus on developing new products as well as on further developing our existing portfolio. This industry in particular is characterized by technological innovations. Digitalization is a more crucial factor here than in any of our other product segments. Devices not only have to be continuously developed in terms of their application, but they also increasingly have to be embedded in the IT system landscape of hospitals, blood donation centers, and plasma centers. In the future, we want to benefit from this trend and are already focusing on the continuous development of our software solutions to increase the efficiency and benefit for our customers.
As part of Fresenius Kabi’s acquisition of Ivenix, a specialized infusion therapy company, Fresenius Kabi’s research and development activities focus on further developing the Infusion Management Systems (IMS) and their software, in particular regarding cybersecurity, workflow optimization, and connectivity with various electronic medical record (EMR) systems.
We also continued the development work on our new infusion management system Exelia in the reporting year. This system features a modern operating system and will enable new therapy and treatment procedures in the intensive care unit and operating room. Fresenius Kabi will continue to develop the Exelia system further to continuously meet the advancing demands in the area of application.
In the reporting year, we continued to develop the Vigilant Software Suite, a software solution for our Agilia family of infusion pumps in hospitals.
In research and development in the area of transfusion technology, we are working intensively on products for use in cell therapy. Our focus is on product developments for the automated washing and concentration of cell concentrates. These products are used in CAR T-cell1 and similar cell therapies. In 2022, we successfully launched the CUE1 cell processing system. This device has been specifically developed for smaller filling quantities and end-use applications in the area of cell therapy and will complement our LOVO1 cell processing system, which is already available on the market.
In the area of extracorporeal photopheresis (ECP), we continue to focus on the introduction of the Amicus Blue system and the associated Phelix light box in Europe, as well as on the further development of an ECP application method, which only requires one vascular access. In this therapy method, certain blood cells outside the body are treated with ultraviolet light (phototherapy). This method is used to treat various immunological diseases, including to kill malignant immune cells (lymphocytes) outside the body.
1 For more information, see the glossary
In the area of generic IV drugs, we are continuously working on the extension of our product portfolio. For example, in the reporting year, we launched the drug Romidepsin injection as an extension of our oncology portfolio in the United States. The product was the only generic for the originator product ISTODAX on the market. Furthermore, the products Bortezomib and Pemetrexed were launched with the formation of the generic market for these products in the United States.
In addition, we are working on the continuous improvement of IV drugs already on the market. For example, we are developing IV drugs with new formulations and dosage forms, as well as improved primary packaging. In 2022, we had about 100 active projects in the area of generics. Our research and development activities focus on complex formulations, such as an emulsion solution that has already been confirmed as a first-to-file abbreviated new drug application (ANDA) submission in the United States, as well as peptide formulations that can be applied with an autoinjector, among other methods. In addition, we are constantly working on product improvements that bring additional benefits to both medical personnel and patients.
For example, we develop ready-to-use products that are especially convenient and safe and help to prevent application errors in day-to-day medical care. These include ready-to-use solutions in our freeflex infusion bags, the cost-effective KabiPac infusion bottle, and prefilled syringes. Drugs in pre-filled syringes are easier and safer to use than traditional applications. In the reporting year, we launched Calcium Gluconate in freeflex bags and Glycopyrrolate in pre-filled syringes in the United States. In Europe, we launched, among other drugs, Icatibant in prefilled syringes. Further, we launched the drug Dexamethasone, which is essential for the treatment of COVID-19 patients, in additional European countries.
To improve drug safety, Fresenius Kabi is implementing a global program to introduce data matrix barcodes on our generic drugs. This initiative is intended to prevent errors in the manual entry of drug information in data management systems, e.g., those of hospitals.
In the area of infusion solutions, we have focused our development activities on improving and developing new container technologies to improve the daily work routine and safety of healthcare professionals, as we have done in previous years. With freeflex+, we further rolled out a needle-free injection port and are working on new projects to complement this container range. Our portfolio extension to the U.S. market is advancing, as we are in the last stages of completing the rollout of our product offering for infusion solutions, which was specifically designed for this key market.

